762016
3-Azido-1-propanamine
≥95%
Sinónimos:
3-Azidopropylamine
About This Item
Productos recomendados
Quality Level
assay
≥95%
form
liquid
reaction suitability
reaction type: click chemistry
refractive index
n20/D 1.471
density
1.020 g/mL at 25 °C
storage temp.
−20°C
SMILES string
NCCCN=[N+]=[N-]
InChI
1S/C3H8N4/c4-2-1-3-6-7-5/h1-4H2
InChI key
OYBOVXXFJYJYPC-UHFFFAOYSA-N
General description
- Bismethylolpropionic acid (bis-MPA) monomers with azide functional group to generate high-generation dendrimers.,
- Clickable zinc tetraphenylporphyrin scaffold with an azido group through click chemistry applicable in photodynamic therapy.
Application
signalword
Danger
hcodes
Hazard Classifications
Acute Tox. 3 Oral - Flam. Liq. 3
Storage Class
3 - Flammable liquids
wgk_germany
WGK 3
flash_point_f
140.0 °F
flash_point_c
60 °C
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
Explore the principles and applications of click chemistry in drug discovery, highlighting efficient reactions that streamline the synthesis of bioactive compounds.
Explore the principles and applications of click chemistry in drug discovery, highlighting efficient reactions that streamline the synthesis of bioactive compounds.
Explore the principles and applications of click chemistry in drug discovery, highlighting efficient reactions that streamline the synthesis of bioactive compounds.
Explore the principles and applications of click chemistry in drug discovery, highlighting efficient reactions that streamline the synthesis of bioactive compounds.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico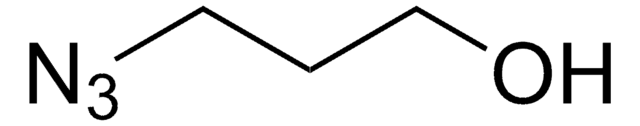


![2-[2-(2-Azidoethoxy)ethoxy]ethanol solution ~0.5 M in tert-butyl methyl ether](/deepweb/assets/sigmaaldrich/product/structures/374/007/eea7ca74-41e4-4aac-af71-c93c37ec0a5a/640/eea7ca74-41e4-4aac-af71-c93c37ec0a5a.png)