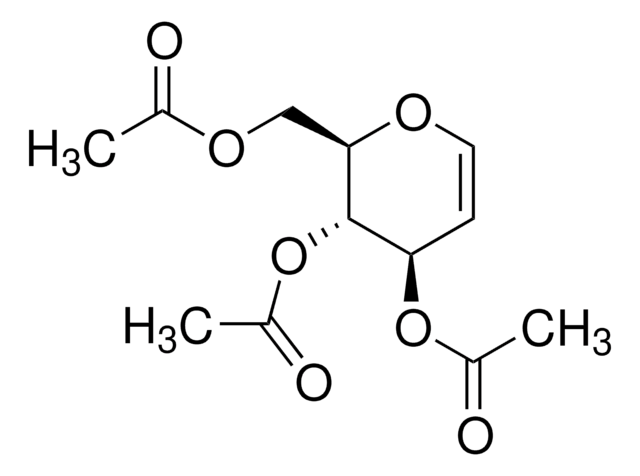
464058
D-Glucal
96%
Sinónimos:
1,5-Anhydro-2-deoxy-D-arabino-hex-1-enitol
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula empírica (notación de Hill):
C6H10O4
Número de CAS:
Peso molecular:
146.14
EC Number:
MDL number:
UNSPSC Code:
12352201
PubChem Substance ID:
NACRES:
NA.22
Productos recomendados
Quality Level
assay
96%
form
solid
optical activity
[α]21/D −7°, c = 1.9 in H2O
impurities
<1% methyl alcohol
mp
58-60 °C (lit.)
storage temp.
2-8°C
SMILES string
OC[C@H]1OC=C[C@@H](O)[C@@H]1O
InChI
1S/C6H10O4/c7-3-5-6(9)4(8)1-2-10-5/h1-2,4-9H,3H2/t4-,5-,6+/m1/s1
InChI key
YVECGMZCTULTIS-PBXRRBTRSA-N
Categorías relacionadas
Application
Important building block for both solution- and solid-phase synthesis of oligosaccharides.
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
A possible catalytic role of pyridoxal-5'-phosphate in a general acid base catalyzed reaction of D-glucal with glycogen phosphorylases.
E J Helmreich et al.
Progress in clinical and biological research, 102 Pt C, 23-35 (1982-01-01)
Syed Khalid Yousuf et al.
The Journal of organic chemistry, 75(9), 3097-3100 (2010-04-13)
Novel one-pot three- and four-component transformations of D-glucal to furan-based hydroxy triazole glycoconjugates have been achieved by sequential addition of reagents in the presence of Cu(OTf)(2)-Cu powder as catalysts. In general the carbohydrate-derived products were formed with high diastereomeric purity.
Russell J Hewitt et al.
The Journal of organic chemistry, 75(3), 955-958 (2010-01-14)
The conversion of cyclopropane-fused carbohydrates into oxepines is an attractive method for accessing diverse members of the septanoside family of carbohydrate mimetics. 2-Bromooxepines are obtained through silver(I)-promoted thermal ring expansion of a d-glucal-derived gem-dihalocyclopropanated sugar. In contrast, cyclopropane ring cleavage
Xi-Kai Cui et al.
Carbohydrate research, 358, 19-22 (2012-08-03)
2-Deoxyglycosides were synthesized in high α-selectivity by the direct addition of alcohols to D-glucal and D-galactal catalyzed by TMSI and PPh(3). The acid labile isopropylidene group is tolerated under this condition.
Russell J Hewitt et al.
Chemical communications (Cambridge, England), 47(1), 421-423 (2010-09-21)
gem-Dibromocyclopropane 1, prepared from tri-O-benzyl-D-glucal, undergoes thermal and silver-promoted ring expansion in the presence of alcohols to give substituted oxepines. With further heating, ring contraction to highly substituted tetrahydrofurans follows. These represent C-furanosides, potentially useful as precursors to C-nucleosides and
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico