160660
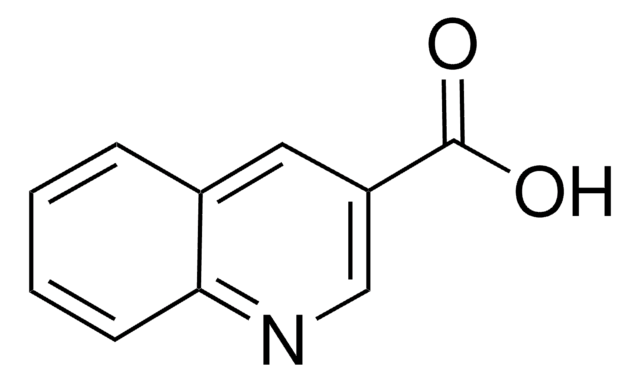
Quinaldic acid
98%
Sinónimos:
2-Quinolinecarboxylic acid
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula empírica (notación de Hill):
C10H7NO2
Número de CAS:
Peso molecular:
173.17
Beilstein/REAXYS Number:
126322
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
form:
solid
assay:
98%
Productos recomendados
Quality Level
assay
98%
form
solid
mp
156-158 °C (lit.)
functional group
carboxylic acid
SMILES string
OC(=O)c1ccc2ccccc2n1
InChI
1S/C10H7NO2/c12-10(13)9-6-5-7-3-1-2-4-8(7)11-9/h1-6H,(H,12,13)
InChI key
LOAUVZALPPNFOQ-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
General description
Quinaldic acid is also referred as quinoline-2-carboxylic acid. Microwave-assisted preparation of substituted anilides of quinaldic acid has been reported. It inhibits the oxidation of pyruvate, α-ketoglutarate, glutamate and citrate in rat liver mitochondria. Quinaldic acid is a metabolite of tryptophan degradation and inhibits the gluconeogenesis in perfused livers.
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Felipe Lombó et al.
Chembiochem : a European journal of chemical biology, 7(2), 366-376 (2006-01-13)
Thiocoraline is a thiodepsipeptide antitumor compound produced by two actinomycetes Micromonospora sp. ACM2-092 and Micromonospora sp. ML1, isolated from two marine invertebrates (a soft coral and a mollusc) found of the Indian Ocean coast of Mozambique. By using oligoprimers derived
B R Bochner et al.
Journal of bacteriology, 143(2), 926-933 (1980-08-01)
A simple technique has been devised that allows direct plate selection of tetracycline-sensitive clones from a predominantly tetracycline-resistant population. The technique is especially useful in genetic methodologies based on the use of tetracycline resistance transposons, such as Tn10. Potential uses
Alleyn T Plowright et al.
Chemistry & biology, 9(5), 607-618 (2002-05-29)
Saframycin A (SafA) is a natural product that inhibits human cancer cell proliferation. Its synthetic analog, QAD, is a more potent inhibitor of these cells. SafA does not affect wild-type yeast, but it does inhibit growth of the strain CCY333
Induction of ornithine decarboxylase activity in mouse urinary bladder by L-tryptophan and some of its metabolites.
M Matsushima et al.
Cancer research, 42(9), 3587-3591 (1982-09-01)
Graham Smith et al.
Acta crystallographica. Section C, Crystal structure communications, 64(Pt 3), o180-o183 (2008-03-07)
The structures of the 1:1 proton-transfer compounds of 4,5-dichlorophthalic acid with 8-hydroxyquinoline, 8-aminoquinoline and quinoline-2-carboxylic acid (quinaldic acid), namely anhydrous 8-hydroxyquinolinium 2-carboxy-4,5-dichlorobenzoate, C(9)H(8)NO(+) x C(8)H(3)Cl(2)O(4)(-), (I), 8-aminoquinolinium 2-carboxy-4,5-dichlorobenzoate, C(9)H(9)N(2)(+) x C(8)H(3)Cl(2)O(4)(-), (II), and the adduct hydrate 2-carboxyquinolinium 2-carboxy-4,5-dichlorobenzoate quinolinium-2-carboxylate monohydrate
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico