All Photos(1)
About This Item
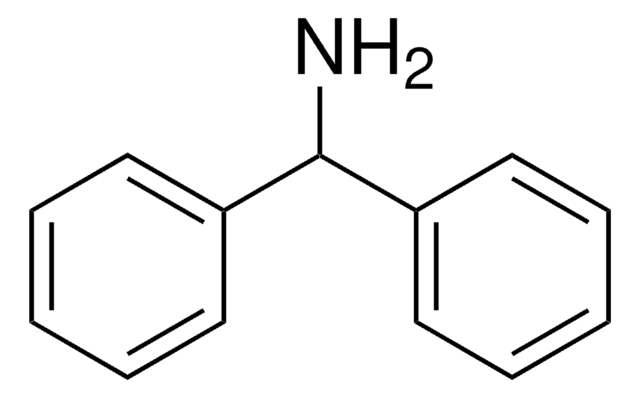
Linear Formula:
(C6H5)2C=NH
CAS Number:
Molecular Weight:
181.23
Beilstein:
1100371
MDL number:
UNSPSC Code:
12171500
PubChem Substance ID:
NACRES:
NA.47
Recommended Products
Quality Level
Assay
95%
form
liquid
refractive index
n20/D 1.618 (lit.)
bp
151-153 °C/10 mmHg (lit.)
density
1.08 g/mL at 25 °C (lit.)
application(s)
diagnostic assay manufacturing
hematology
histology
storage temp.
room temp
SMILES string
N=C(c1ccccc1)c2ccccc2
InChI
1S/C13H11N/c14-13(11-7-3-1-4-8-11)12-9-5-2-6-10-12/h1-10,14H
InChI key
SXZIXHOMFPUIRK-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Benzophenone imine plays a vital role as an ammonia surrogate. It is primarily used as ammonia equivalents for the selective formation of protected primary amines. Benzophenone imine is useful as a synthetic intermediate, especially for the synthesis of glycine Schiff base.
Application
Benzophenone imine is useful for the preparation of nitrile yield dimers.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
228.2 °F - closed cup
Flash Point(C)
109 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Suzanne Fergus et al.
The Journal of organic chemistry, 69(14), 4663-4669 (2004-07-03)
A series of novel hexaaryl diazatrienes 5 ("nitrile ylide dimers") were synthesized directly from the corresponding diaryl ketimines 12 and dichlorotoluenes 13 in a facile one-pot synthesis. The carbene character of the nitrile ylides was investigated by varying the substituents
Lukas Lohmeyer et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 26(26), 5834-5845 (2020-02-06)
New redox-active 1,2,5,6-tetrakis(guanidino)-naphthalene compounds, isolable and storable in the neutral and deep-green dicationic redox states and oxidisable further in two one-electron steps to the tetracations, are reported. Protonation switches on blue fluorescence, with the fluorescence intensity (quantum yield) increasing with
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service