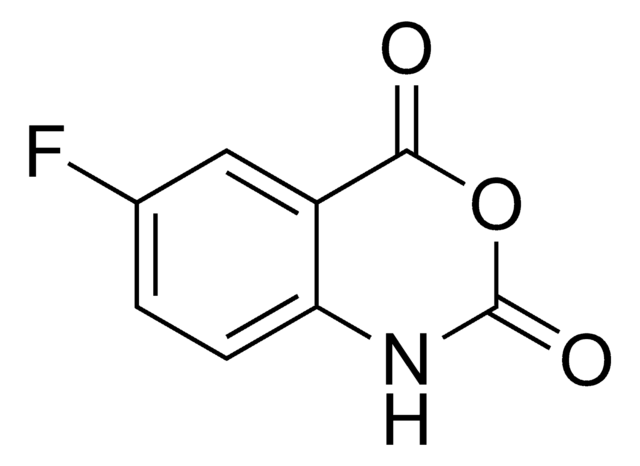
I12808
Isatoic anhydride
96%
Synonym(s):
3,1-Benzoxazine-2,4(1H)-dione, Anthranilic acid N-carboxylic acid anhydride
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C8H5NO3
CAS Number:
Molecular Weight:
163.13
Beilstein:
136786
EC Number:
MDL number:
UNSPSC Code:
12162002
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
vapor density
5.6 (vs air)
Quality Level
Assay
96%
mp
233 °C (dec.) (lit.)
SMILES string
O=C1Nc2ccccc2C(=O)O1
InChI
1S/C8H5NO3/c10-7-5-3-1-2-4-6(5)9-8(11)12-7/h1-4H,(H,9,11)
InChI key
TXJUTRJFNRYTHH-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Sens. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
586.4 °F - closed cup
Flash Point(C)
308 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
M G Page
The Biochemical journal, 295 ( Pt 1), 295-304 (1993-10-01)
Class C beta-lactamases from Pseudomonas aeruginosa and several species of the Enterobacteriaceae have been observed to undergo a rapid burst in hydrolysis of beta-lactam antibiotics before relaxation to a steady-state rate of hydrolysis. The amplitude of the burst corresponds to
Christine Hiu-Tung Chen et al.
Molecular diversity, 9(4), 353-359 (2005-11-29)
Reactions using fluorous reagents and scavengers are compared side-by-side with their solid-supported counterparts. Fluorous triphenylphosphine is used in the bromination reaction of alcohols, fluorous thiol is used as an electrophile scavenger for alpha-bromoketones, fluorous isatoic anhydride is used as a
J E Churchich
Analytical biochemistry, 213(2), 229-233 (1993-09-01)
Isatoic anhydride reacts with nucleophile groups of proteins to yield o-aminobenzoyl protein conjugates. The fluorescence emitted by the chromophore decays in a multiexponential manner with average fluorescence lifetimes ranging from 9.9 to 10.7 ns. The steady emission anisotropy, measured upon
Zhan-Hui Zhang et al.
Journal of combinatorial chemistry, 12(5), 643-646 (2010-08-06)
A simple and efficient protocol for one-pot three-component coupling of isatoic anhydride, amines, and aldehydes in water using magnetically recoverable Fe(3)O(4) nanoparticles is reported. This methodology results in the synthesis of a variety of 2,3-dihydroquinazolin-4(1H)-ones in high yields. The catalyst
M H Gelb et al.
Journal of medicinal chemistry, 29(4), 585-589 (1986-04-01)
Derivatives of isatoic anhydride were prepared and tested as inhibitors of serine proteases. A number of isatoic anhydrides with positively charged substituents irreversibly inactivated several trypsin-like enzymes and preferentially inactivated trypsin over chymotrypsin. Further selectivity was obtained by introduction of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service