425117
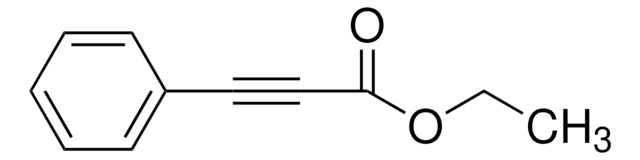
Ethyl 2-butynoate
98%
Synonym(s):
Ethyl tetrolate
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH3C≡CC(O)OCH2CH3
CAS Number:
Molecular Weight:
112.13
Beilstein:
1744948
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
liquid
refractive index
n20/D 1.436 (lit.)
bp
160-161 °C/730 mmHg (lit.)
density
0.962 g/mL at 25 °C (lit.)
functional group
ester
SMILES string
CCOC(=O)C#CC
InChI
1S/C6H8O2/c1-3-5-6(7)8-4-2/h4H2,1-2H3
InChI key
FCJJZKCJURDYNF-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Ethyl 2-butynoate, a 2-alkynoate is a methanogenesis inhibitor. It is an electron deficient internal alkyne that has been reported to undergo codimerization with alkenes to form 1,3-dienes catalyzed by rhodium(I)/H8-BINAP complex. The annulation of thioamides with ethyl 2-butynoate catalyzed by tri-n-butylphosphine to form thiazolines has been investigated.
Application
Ethyl 2-butynoate was used in the study of its effect on in vitro degradation and microbial biomass production.
It may be used in the synthesis of the following:
It may be used in the synthesis of the following:
- 3-ethyl, 1-methyl 1-(2-nitrophenyl)-cyclopent-3-ene-1,3-dicarboxylate
- tricyclic aziridine derivatives
- alkenylsilanols
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
143.6 °F - closed cup
Flash Point(C)
62 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Bing Liu et al.
The Journal of organic chemistry, 67(13), 4595-4598 (2002-06-22)
The annulation of thioamides with 2-alkynoates and 2,3-dienoates under the catalysis of tri-n-butylphosphine was described. The annulation reaction provided a new entry to thiazolines, particularly those with 2-aryl substituents.
Synthesis of 2-azaspiro [4.4] nonan-1-ones via phosphine-catalysed [3+ 2]-cycloadditions.
Yong SR, et al.
Tetrahedron, 61(34), 8120-8129 (2005)
Yu Shibata et al.
Organic letters, 10(13), 2829-2831 (2008-06-12)
A cationic rhodium(I)/H(8)-BINAP complex catalyzes codimerization of alkenes bearing no alpha-hydrogen and electron-deficient internal alkynes, leading to 1,3-dienes in good yields with moderate to excellent regio- and stereoselectivity. The same complex also catalyzes codimerization of an acrylate and phenyl-substituted electron-rich
Dichloro [TADDOLato (2-)-O,O'] titanium/Dichlorobis [1-methylethoxy] titanium-Mediated, Highly Diastereo-and Enantioselective Additions of Silyl Enol Ethers to Nitro Olefins and [3+ 2] Cycloadditions of Primary Adducts to Acetylenes.
Seebach D, et al.
Helvetica Chimica Acta, 82(11), 1829-1842 (1999)
Efficient and stereoselective cross-coupling with highly substituted alkenylsilanols.
Denmark SE and Pan W.
Journal of Organometallic Chemistry, 653(1), 98-104 (2002)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service



![1,8-Diazabicyclo[5.4.0]undec-7-ene 98%](/deepweb/assets/sigmaaldrich/product/structures/120/564/5b373e23-1624-489c-8efb-692de0f96ffb/640/5b373e23-1624-489c-8efb-692de0f96ffb.png)







