All Photos(1)
About This Item
Linear Formula:
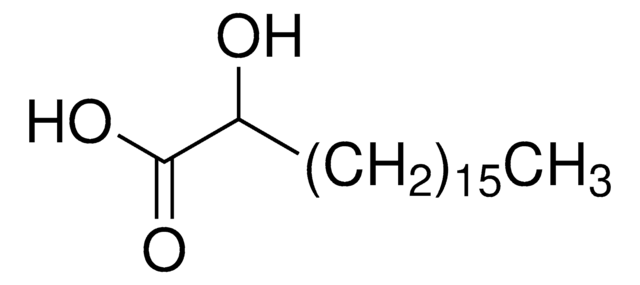
HO(CH2)14CO2H
CAS Number:
Molecular Weight:
258.40
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
mp
85-89 °C (lit.)
SMILES string
OCCCCCCCCCCCCCCC(O)=O
InChI
1S/C15H30O3/c16-14-12-10-8-6-4-2-1-3-5-7-9-11-13-15(17)18/h16H,1-14H2,(H,17,18)
InChI key
BZUNJUAMQZRJIP-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
15-Hydroxypentadecanoic acid is an ω-hydroxy acid. One of the method reported for its synthesis is from 1,12-dodecanolide. It is reported to be one of the bioactive component in Tagetes erecta L. leaf and flower extract.
15-Hydroxypentadecanoic acid undergoes lactonization reaction catalyzed by Mucor javanicus L46 and Mucor miehei to afford macrocyclic mono- and oligolactone derivatives. Its lipase-catalyzed synthesis from 15-tetracosenoic acid in Malania Olcifera Chum oil has been proposed. It also participates in the biosynthesis of pentadecanolide.
Application
15-Hydroxypentadecanoic acid is suitable reagent used in the following studies:
- As an internal standard in the quantification of formation of 11-hydroxylauric acid by gas chromatography.
- In the synthesis of [16-14C]16DCA (DCA= dicarboxylic acid) by one-carbon elongation procedure at C15.
- As an internal standard for the normalization of intensities in the mass spectra of plant cutin polymer.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Enzymatic lactonization of 15-hydroxypentadecanoic and 16-hydroxyhexadecanoic acids to macrocyclic lactones.
Antczak U, et al.
Enzyme and Microbial Technology, 13(7), 589-593 (1991)
Lipase catalyzed synthesis of pentadecanolide from 15-hydroxypentadecanoic acid.
Pan XB, et al.
Chinese Journal of Applied Chemistry / Ying Yong Hua Xue, 21(8), 850-852 (2004)
Sacha Ferdinandusse et al.
Journal of lipid research, 45(6), 1104-1111 (2004-04-03)
Dicarboxylic acids (DCAs) are omega-oxidation products of monocarboxylic acids. After activation by a dicarboxylyl-CoA synthetase, the dicarboxylyl-CoA esters are shortened via beta-oxidation. Although it has been studied extensively where this beta-oxidation process takes place, the intracellular site of DCA oxidation
Jamal Mustafa et al.
Lipids, 39(2), 167-172 (2004-05-12)
Derivatives of podophyllotoxin were prepared by coupling 10 FA with the C4-alpha-hydroxy function of podophyllotoxin. The coupling reactions between FA and podophyllotoxin were carried out by dicyclohexylcarbodiimide in the presence of a catalytic amount of dimethylaminopyridine to produce quantitative yields
Preparation of 15-hydroxypentadecanoic acid by means of condensation reaction via β-ketosulfoxide.
Nozaki H, et al.
Canadian Journal of Chemistry, 46(23), 3767-3770 (1968)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service

![2-[2-(2-Methoxyethoxy)ethoxy]acetic acid technical grade](/deepweb/assets/sigmaaldrich/product/structures/335/694/b58c539b-141f-4ab2-98d9-5f46c748490b/640/b58c539b-141f-4ab2-98d9-5f46c748490b.png)