340928
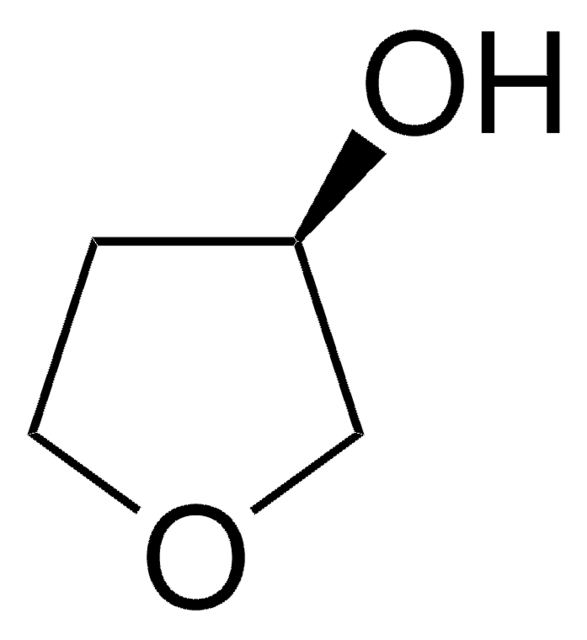
1,4-Anhydroerythritol
95%
Synonym(s):
(3R,4S)-Tetrahydro-3,4-furandiol, Erythritol-1,4-anhydride, cis-3,4-Dihydroxytetrahydrofuran, cis-Tetrahydrofuran-3,4-diol, meso-3,4-Dihydroxytetrahydrofuran, rel-(3R,4S)-Tetrahydro-3,4-furandiol
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C4H8O3
CAS Number:
Molecular Weight:
104.10
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
95%
form
liquid
refractive index
n20/D 1.477 (lit.)
density
1.27 g/mL at 25 °C (lit.)
functional group
ether
hydroxyl
SMILES string
O[C@H]1COC[C@H]1O
InChI
1S/C4H8O3/c5-3-1-7-2-4(3)6/h3-6H,1-2H2/t3-,4+
InChI key
SSYDTHANSGMJTP-ZXZARUISSA-N
General description
1,4-Anhydroerythritol is a cyclic vicinal diol. 1,4-Anhydroerythritol undergoes hydrodeoxygenation over tungsten oxide-palladium catalysts to yield 3-hydroxytetrahydrofuran.
Application
1,4-Anhydroerythritol was employed in a key step in the synthesis of glucopyranoside-based analogs of adenophostin A lacking the adenine component.
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Yasushi Amada et al.
ChemSusChem, 7(8), 2185-2192 (2014-07-01)
Hydrodeoxygenation of cyclic vicinal diols such as 1,4-anhydroerythritol was conducted over catalysts containing both a noble metal and a group 5-7 transition-metal oxide. The combination of Pd and WOx allowed the removal of one of the two OH groups selectively.
Aritomo Yamaguchi et al.
Physical chemistry chemical physics : PCCP, 19(4), 2714-2722 (2016-11-30)
The intramolecular dehydration of biomass-derived sugar alcohols d-sorbitol, d-mannitol, galactitol, xylitol, ribitol, l-arabitol, erythritol, l-threitol, and dl-threitol was investigated in high-temperature water at 523-573 K without the addition of any acid catalysts. d-Sorbitol and d-mannitol were dehydrated into isosorbide and
Alex C Watanabe et al.
Environmental science & technology, 52(15), 8346-8354 (2018-06-29)
Laboratory and field measurements have demonstrated that isoprene epoxydiol (IEPOX) is the base component of a wide range of chemical species found in isoprene-derived secondary organic aerosol (SOA). To address newly raised questions concerning the chemical identities of IEPOX-derived SOA
Andrew M Riley et al.
Carbohydrate research, 337(12), 1067-1082 (2002-06-14)
Adenophostins A and B are naturally occurring glyconucleotides that interact potently with receptors for D-myo-inositol 1,4,5-trisphosphate, an important second messenger molecule in most cell types. Here we describe the design and synthesis of glucopyranoside-based analogues of adenophostin A lacking the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service