All Photos(1)
About This Item
Empirical Formula (Hill Notation):
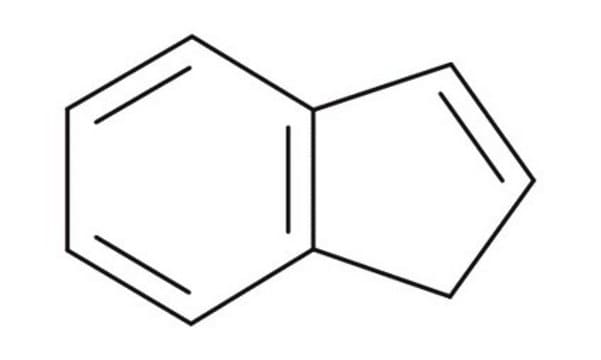
C9H8
CAS Number:
Molecular Weight:
116.16
Beilstein:
635873
EC Number:
MDL number:
UNSPSC Code:
12352100
eCl@ss:
39011614
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
liquid
refractive index
n20/D 1.595 (lit.)
bp
181-182 °C (lit.)
mp
−5-−3 °C (lit.)
solubility
organic solvents: miscible
water: insoluble
density
0.996 g/mL at 25 °C (lit.)
storage temp.
2-8°C
SMILES string
C1C=Cc2ccccc12
InChI
1S/C9H8/c1-2-5-9-7-3-6-8(9)4-1/h1-6H,7H2
InChI key
YBYIRNPNPLQARY-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Indene is oxidized to mixtures of cis- and trans-indandiols and related metabolites by Pseudomonas putida and Rhodococcus sp.
Application
Indene was used in the synthesis of new C60 derivative, indene-C60 bisadduct. It was used in preparing polyindene by the controlled cationic polymerization initiated with cumyl methyl ether/TiCl4 in CH2Cl2.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Asp. Tox. 1 - Flam. Liq. 3
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
136.4 °F - closed cup
Flash Point(C)
58 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
B C Buckland et al.
Metabolic engineering, 1(1), 63-74 (2000-08-10)
Indene is oxidized to mixtures of cis- and trans-indandiols and related metabolites by Pseudomonas putida and Rhodococcus sp. isolates. Indene metabolism is consistent with monooxygenase and dioxygenase activity. P. putida resolves enantiomeric mixtures of cis-1,2-indandiol by further selective oxidation of
High glass transition temperature polyolefins obtained by the catalytic hydrogenation of polyindene.
Hahn SF and Hillmyer MA.
Macromolecules, 36(1), 71-76 (2003)
Lucas J Gursky et al.
Applied microbiology and biotechnology, 85(4), 995-1004 (2009-07-02)
The styAB genes from Pseudomonas putida CA-3, which encode styrene monooxygenase, were subjected to three rounds of in vitro evolution using error-prone polymerase chain reaction with a view to improving the rate of styrene oxide and indene oxide formation. Improvements
Regioselective synthesis of indenols by rhodium-catalyzed C-H activation and carbocyclization of aryl ketones and alkynes.
Krishnamoorthy Muralirajan et al.
Angewandte Chemie (International ed. in English), 50(18), 4169-4172 (2011-03-31)
Adam C Glass et al.
Organic letters, 10(21), 4855-4857 (2008-10-07)
A new methodology for the preparation of substituted naphthalenes starting from readily available indenones, organometal reagents, and trimethylsilyldiazomethane via a catalytic rearrangement process is described. Hindered biaryl naphthalenes, including triortho-substituted biaryls, can be accessed through our method. Our results are
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service










![Dichloro[rac-ethylenebis(indenyl)]zirconium(IV)](/deepweb/assets/sigmaaldrich/product/structures/296/699/b249f923-58d9-45b3-bd63-b110942453d3/640/b249f923-58d9-45b3-bd63-b110942453d3.png)