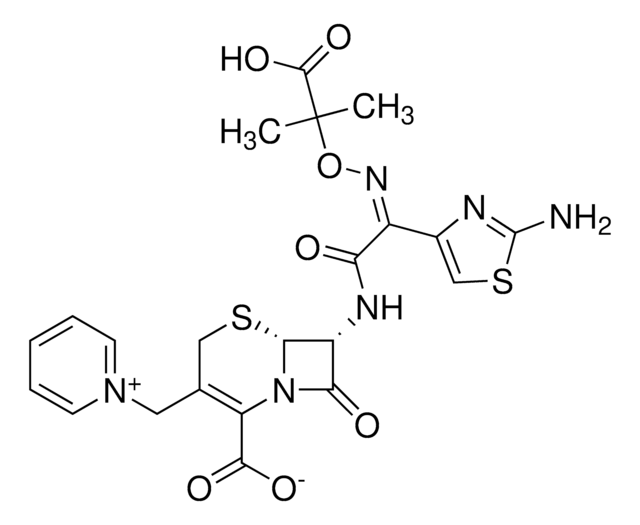
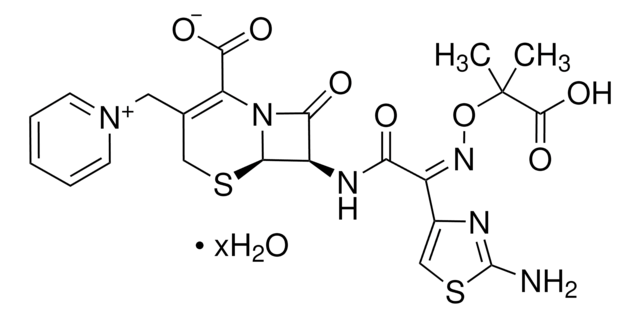
The solubility of Ceftazidime hydrate (C3809) is as follows: it is soluble at 50mg/mL in 0.1 M NaOH.
C3809
Ceftazidime hydrate
90.0-105.0%, contains ~10% sodium carbonate as stabilizer
Synonym(s):
1-[[(6R,7R)-7-[[(2Z)-(2-amino-4-thiazolyl)[(1-carboxy-1-methylethoxy)imino]acetyl]amino]-2-carboxy-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-en-3-yl]methyl]pyridinum
Select a Size
Select a Size
About This Item
Recommended Products
Quality Level
Assay
90.0-105.0%
form
powder or crystals
contains
~10% sodium carbonate as stabilizer
antibiotic activity spectrum
Gram-negative bacteria
Gram-positive bacteria
Mode of action
cell wall synthesis | interferes
storage temp.
2-8°C
SMILES string
[H]O[H].[H]O[H].[H]O[H].[H]O[H].[H]O[H].CC(C)(O\N=C(/C(=O)N[C@H]1[C@H]2SCC(C[n+]3ccccc3)=C(N2C1=O)C([O-])=O)c4csc(N)n4)C(O)=O
InChI
1S/C22H22N6O7S2.5H2O/c1-22(2,20(33)34)35-26-13(12-10-37-21(23)24-12)16(29)25-14-17(30)28-15(19(31)32)11(9-36-18(14)28)8-27-6-4-3-5-7-27;;;;;/h3-7,10,14,18H,8-9H2,1-2H3,(H4-,23,24,25,29,31,32,33,34);5*1H2/b26-13-;;;;;/t14-,18-;;;;;/m1...../s1
InChI key
NMVPEQXCMGEDNH-TZVUEUGBSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
Biochem/physiol Actions
Other Notes
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Resp. Sens. 1 - Skin Sens. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
-
What is the recommended method for reconstituting the product C3809, Ceftazidime hydrate?
1 answer-
Helpful?
-
-
What is the solution stability of the Product C3809, Ceftazidime hydrate?
1 answer-
Unfortunately, we do not test Product C3809, Ceftazidime hydrate, for solution stability. However, Chedru-Legros V. et al., Cornea, 29(7),807-11(2010), report 5% solutions of ceftazidime 0.9% sodium chloride can be stored at -20°C for 6 months. Also, Mehta, S. et al., Retina, 31(7),1316-22 (2011), reports single-use polypropylene syringes retain potency, sterility, and stability for 24 weeks when stored at -20°C or -80°C.
Helpful?
-
-
What is the Department of Transportation shipping information for this product?
1 answer-
Transportation information can be found in Section 14 of the product's (M)SDS.To access the shipping information for this material, use the link on the product detail page for the product.
Helpful?
-
-
How does the storage temperature relate to shipping conditions?
1 answer-
The storage conditions that a Sigma-Aldrich catalog and label recommend for products are deliberately conservative. For many products, long-term storage at low temperatures will increase the time during which they are expected to remain in specification and therefore are labeled accordingly. Where short-term storage, shipping time frame, or exposure to conditions other than those recommended for long-term storage will not affect product quality, Sigma-Aldrich will ship at ambient temperature. The products sensitive to short-term exposure to conditions other than their recommended long-term storage are shipped on wet or dry ice. Ambient temperature shipping helps to control shipping costs for our customers. At any time, our customers can request wet- or dry-ice shipment, but the special handling is at customer expense if our product history indicates that the product is stable for regular shipment.
Helpful?
-
Active Filters
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service