SMB01381
SAICAriboside
≥95% (HPLC), from synthetic, solid
Synonym(s):
N-Succinyl-5-aminoimidazole-4-carboxamide Ribose
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C13H18N4O9
CAS Number:
Molecular Weight:
374.30
MDL number:
UNSPSC Code:
12352211
UNSPSC Code:
12352200
NACRES:
NA.26
Recommended Products
biological source
synthetic
Quality Level
Assay
≥95% (HPLC)
form
solid
color
white to beige
mp
130-135 °C
storage temp.
2-8°C
SMILES string
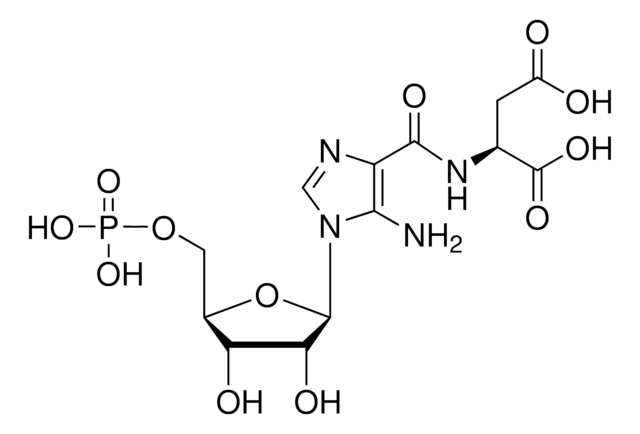
NC1=C(C(N[C@H](C(O)=O)CC(O)=O)=O)N=CN1[C@H]2[C@H](O)[C@H](O)[C@@H](CO)O2
General description
SAICAR (a ribotide) can lose its phosphate group leading to the appearance of a riboside known as succinylaminoimidazolecarboxamide riboside (SAICAriboside or SAICAr) in cerebrospinal fluid, in urine, and, to a lesser extent, in plasma. SAICAriboside is characteristic of ADSL, a heritable deficiency of the enzyme adenylosuccinate lyase (ASL or adenylosuccinase) responsible for metabolizing SAICAR (SZMP) to AICAR (ZMP) and adenylosuccinate (SAMP) to AMP. ASL deficiency causes increased SAICAR & SAMP, and their corresponding rephosphorylated products SAICAr & succinyladenosine (S-Ado).
Storage and Stability
Heat sensitive
related product
Product No.
Description
Pricing
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Study of purinosome assembly in cell-based model systems with de novo purine synthesis and salvage pathway deficiencies
Baresova, et al.
PLoS ONE, 13, e0201432-e0201432 (2018)
A mild form of adenylosuccinate lyase deficiency in absence of typical brain MRI features diagnosed by whole exome sequencing
Macchiaiolo, et al.
Italian Journal of Pediatrics, 43, 65-65 (2017)
Delphine C Douillet et al.
The Journal of biological chemistry, 294(3), 805-815 (2018-11-28)
5-Aminoimidazole-4-carboxamide 1-β-d-ribofuranoside (AICAR, or acadesine) is a precursor of the monophosphate derivative 5-amino-4-imidazole carboxamide ribonucleoside 5'-phosphate (ZMP), an intermediate in de novo purine biosynthesis. AICAR proved to have promising anti-proliferative properties, although the molecular basis of its toxicity is poorly
Mass spectrometric analysis of purine de novo biosynthesis intermediates
Madrova, et al.
PLoS ONE, 13, e0208947-e0208947 (2018)
Roxane Marsac et al.
Genetics, 211(4), 1297-1313 (2019-02-01)
Purine homeostasis is ensured through a metabolic network widely conserved from prokaryotes to humans. Purines can either be synthesized de novo, reused, or produced by interconversion of extant metabolites using the so-called recycling pathway. Although thoroughly characterized in microorganisms, such
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service