925225
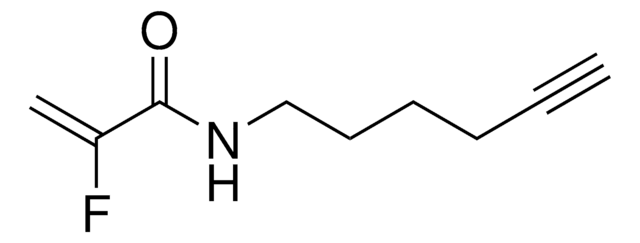
KB02yne
Synonym(s):
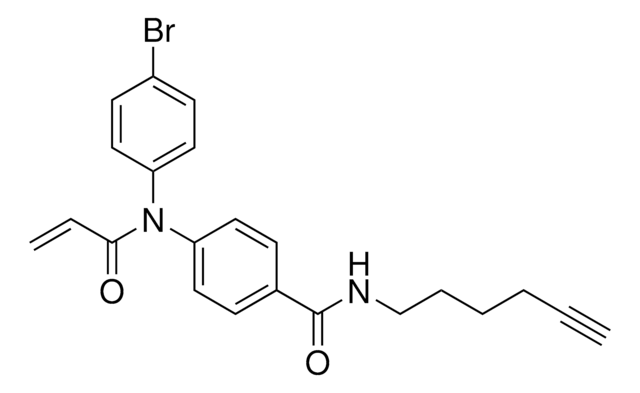
2-Chloro-1-(6-(hex-5-yn-1-yloxy)-3,4-dihydroquinolin-1(2H)-yl)ethan-1-one, Functionalized scout fragment
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C17H20ClNO2
CAS Number:
Molecular Weight:
305.80
UNSPSC Code:
12352101
NACRES:
NA.22
Recommended Products
form
powder or crystals (or Solid or Liquid)
Quality Level
reaction suitability
reagent type: chemical modification reagent
reaction type: click chemistry
storage temp.
2-8°C
Related Categories
Application
KB02yne is a cysteine-reactive small-molecule fragment for chemoproteomic and ligandability studies for both traditionally druggable proteins as well as ″undruggable,″ or difficult-to-target, proteins. This fragment electrophile is the functionalized version of KB02 (912131).
Related useful products may include:
Technology spotlight: Proteomic Ligandability Assessment
Related useful products may include:
- Cysteine-reactive fragments: KB02 (912131), KB03 (912654), KB05 (911798), sulfoxide (925136), CoLDR probe (923818)
- Functionalized scout fragments: KB02-COOH (925047), KB05yne (925144)
- Electrophilc degraders featuring scout fragments: KB02-SLF (914738), KB03-SLF (914975), KB05-SLF (913715), Biotin-SLF (914223)
- Cysteine-reactive probes for chemoproteomics: IA alkyne (924237), IA 5-TAMRA (925020), desthiobiotin iodoacetamide (923826), or biotin iodoacetamide (B2059)
Technology spotlight: Proteomic Ligandability Assessment
related product
Product No.
Description
Pricing
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Vincent M Crowley et al.
ACS central science, 7(4), 613-623 (2021-06-01)
Covalent ligands are a versatile class of chemical probes and drugs that can target noncanonical sites on proteins and display differentiated pharmacodynamic properties. Chemical proteomic methods have been introduced that leverage electrophilic fragments to globally profile the covalent ligandability of
Kristine Senkane et al.
Angewandte Chemie (International ed. in English), 58(33), 11385-11389 (2019-06-22)
Reversible covalency, achieved with, for instance, highly electron-deficient olefins, offers a compelling strategy to design chemical probes and drugs that benefit from the sustained target engagement afforded by irreversible compounds, while avoiding permanent protein modification. Reversible covalency has mainly been
Xiaoyu Zhang et al.
Nature chemical biology, 15(7), 737-746 (2019-06-19)
Ligand-dependent protein degradation has emerged as a compelling strategy to pharmacologically control the protein content of cells. So far, however, only a limited number of E3 ligases have been found to support this process. Here, we use a chemical proteomic
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service