317047
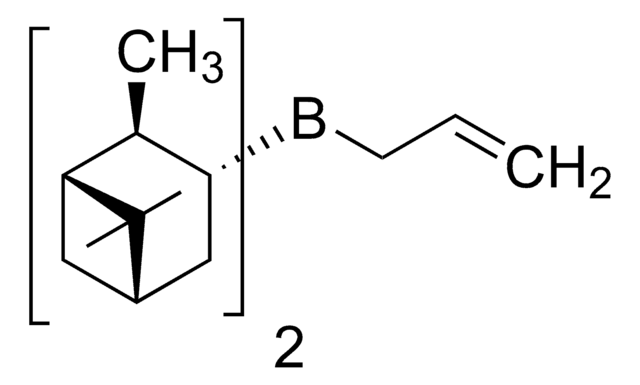
(−)-B-Methoxydiisopinocampheylborane
Synonym(s):
(−)-Diisopinocampheylmethoxyborane
Sign Into View Organizational & Contract Pricing
All Photos(4)
About This Item
Empirical Formula (Hill Notation):
C21H37BO
CAS Number:
Molecular Weight:
316.33
MDL number:
UNSPSC Code:
12352001
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
form
solid
Quality Level
SMILES string
COB([C@H]1CC2CC([C@@H]1C)C2(C)C)[C@H]3CC4CC([C@@H]3C)C4(C)C
InChI
1S/C21H37BO/c1-12-16-8-14(20(16,3)4)10-18(12)22(23-7)19-11-15-9-17(13(19)2)21(15,5)6/h12-19H,8-11H2,1-7H3/t12-,13-,14-,15-,16+,17+,18-,19-/m0/s1
InChI key
IAQXEQYLQNNXJC-BAMGFKBFSA-N
General description
B-Methoxydiisopinocampheylborane (Ipc2BOMe) is an organoborane compound, which is prepared from excess α-pinene, borane dimethylsulfide, and methanol via the formation of an intermediate diisocampheylborane. Ipc2BOMe is used as a versatile reagent for the construction of C-C bonds in asymmetric synthesis.
Application
Reactant involved in organic synthesis reactions such as:
- Double allylboration for synthesis of fragments of tetrafibricin
- Anticancer cytotoxic monorhizopodin synthesis
- Annulation of cyclic allylsilanes
- Asymmetric synthesis of β-amino-α-hydroxy acid taxol side chain analogs
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
B-Methoxydiisopinocampheylborane (Ipc2BOMe): A Pinene Based Auxiliary for Asymmetric C-C Bond-Forming Reactions
Hertweck C and Boland W
J. Prakt. Chem., 341(1), 83-87 (1999)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service