All Photos(1)
About This Item
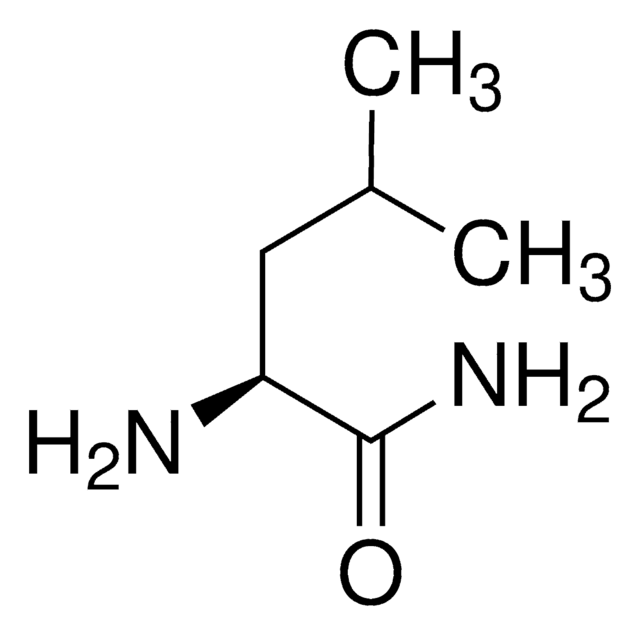
Linear Formula:
(CH3)2CHCH2CH(NH2)CONH2·HCl
CAS Number:
Molecular Weight:
166.65
Beilstein:
4237021
EC Number:
MDL number:
UNSPSC Code:
12352209
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
optical activity
[α]25/D +10°, c = 5 in H2O
reaction suitability
reaction type: solution phase peptide synthesis
mp
254-256 °C (lit.)
application(s)
peptide synthesis
SMILES string
Cl.CC(C)C[C@H](N)C(N)=O
InChI
1S/C6H14N2O.ClH/c1-4(2)3-5(7)6(8)9;/h4-5H,3,7H2,1-2H3,(H2,8,9);1H/t5-;/m0./s1
InChI key
VSPSRRBIXFUMOU-JEDNCBNOSA-N
Looking for similar products? Visit Product Comparison Guide
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
[Leucinamide specific cytosol leucine aminopeptidase].
T Kanno
Rinsho byori. The Japanese journal of clinical pathology, 30(5), 502-506 (1982-05-01)
A conductometric method for the assay of amidase and peptidase activities.
C R Hill et al.
Analytical biochemistry, 120(1), 165-175 (1982-02-01)
Determination of the enantiomers of ketoprofen in blood plasma by ion-pair extraction and high-performance liquid chromatography of leucinamide derivatives.
S Björkman
Journal of chromatography, 414(2), 465-471 (1987-03-06)
Andrew R Conrad et al.
The journal of physical chemistry. A, 115(34), 9676-9681 (2011-05-12)
Rotational spectra were recorded for two isotopic species of two conformers of the amide derivative of leucine in the range of 10.5-21 GHz and fit to a rigid rotor Hamiltonian. Ab initio calculations at the MP2/6-311++G(d,p) level identified the low
Formation of diastereomeric derivatives of 2-arylpropionic acids using L-leucinamide.
H Spahn
Journal of chromatography, 423, 334-339 (1987-12-25)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service