Oligonucleotide Standard 6 Mix LC-UV Analysis
Jessie Zhixin Miao, Geoffrey Rule, Cory Muraco, Uma Sreenivasan
MilliporeSigma
Oligoncleotide

Chromatography

Measurement and Analysis

Introduction
With the COVID-19 pandemic, oligonucleotides (Oligos) have proven their importance in diagnostic and therapeutic applications. Currently, 11 oligonucleotide drugs crossing many disease areas have been approved by the FDA.1, 2 Obstacles preventing quicker development of oligonucleotide therapeutics include the challenges of unfavorable absorption, distribution, metabolism, excretion, and toxicity (ADMET) studies for many clinical trials.2 Some strategies have been developed to tackle the challenges, such as chemical modification to improve drug delivery.
Synthetic oligonucleotides are typically small, single- or double-stranded modified nucleic acids.2 There are many established techniques to analyze and characterize oligonucleotides, including capillary gel electrophoresis (CGE), ion exchange chromatography (IEX), and ion pair reversed-phase liquid chromatography (IP-RPLC). Generally, liquid chromatography is very challenging due to the similarity of oligonucleotide structures, very polar characteristics, presence of truncated and/or modified oligos, ease of self-association into a variety of conformations, and affinity for metal surfaces.1,2 This application describes the separation of an internally produced oligonucleotide standard (Oligo Standard 6) mix, which includes six oligonucleotides, on Chromolith® RP-18e column from the Supelco® portfolio.
Experimental Procedure
Oligo Standard 6 is an internal (in-house) system suitability mix for HPLC-UV evaluation of oligonucleotide separations. It contains six components with molecular weights of 3588.3 Da (Oligo 1), 4157.93 Da (Oligo 2), 7580.83 Da (Oligo 3), 10014.35 Da (Oligo 4), 6116.97 Da (Oligo 5), and 4395.8 Da (Oligo 6) following their elution order on Chromolith® RP-18e columns tested here.
Reagent Preparation
50 mM of Triethylammonium Acetate (TEAA)
To prepare 1 L of 50 mM TEAA, 50 mL of TEAA (commercial 1 M solution) was added into 950 mL of HPLC grade water and mixed well.
20 mM of Triethylammonium Acetate (TEAA)
To prepare 1 L of 20 mM TEAA, 20 mL of TEAA (commercial 1 M solution) was added into 980 mL of HPLC grade water and mixed well.
5 mM of Triethylammonium Acetate (TEAA)
To prepare 1 L of 5 mM TEAA, 5 mL of TEAA (commercial 1 M solution) was added into 995 mL of HPLC grade water and mixed well.
Sample Preparation
5 µM of Oligo Standard 6 sample
1 mL of HPLC grade water was added into the sample vial which contains 5 nmol each of the six oligo components and mixed well.
HPLC-UV System Setup and Data Analysis
Essential settings of the HPLC-UV chromatography system for analysis of Oligo Standard 6 are listed in Table 1 below.
Results and Discussion
With the linkage of phosphate groups, oligonucleotides tend to stick to metal surfaces present in stainless steel column hardware and the LC system, resulting in reduced sensitivity and inaccurate quantitation. Researchers have made a variety of efforts to mitigate this adsorption inside instrumentation, such as treatment of the system with EDTA, high pH mobile phase, or utilizing bio-inert HPLC system components.3 Conventional HPLC columns are typically packed in metal columns, exposing the metal surfaces with the positive charge which can adsorb acidic molecules, such as oligonucleotides containing phosphate groups. Chromolith® HPLC columns are made of highly porous monolithic rods of silica. These columns have an innovative, bimodal pore structure and are packed in metal-free PEEK (polyetheretherketone) columns. This attribute makes them a good candidate for oligonucleotide analysis.
Chromolith® Performance RP-18e, 100 x 4.6 mm column
High Flow Rate Test
To improve separation efficiencies, the particle size of packing material is usually reduced. Currently, conventional HPLC columns contain 5, 3, 2, and even sub 2 µm silica particles. However, the smaller particle size will cause higher back pressure affecting the assay throughput, robustness, and column lifetime. The optimal solution is to use a column that offers faster throughput without the risk of high back pressure. Since the Chromolith® column is not packed with silica particles but a single rod of high-purity, polymeric silica gel, the unique construction enables highly efficient separations at accelerated speeds, which is ideal for high throughput analysis.4
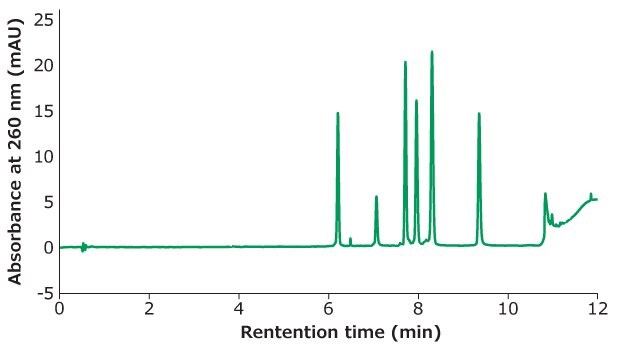
Figure 1.Oligo Standard 6 separation on Chromolith®Performance RP-18e, 100 x 4.6 mm column at flow rate of 3 mL/min with a gradient of 5% B to 15 % B in 10 minutes. Mobile phase A: 50 mM TEAA in water; Mobile phase B: acetonitrile. Injection volume: 5 μL (25 pmol on column).
Figure 1 shows the separation of Oligo Standard 6 on a Chromolith® RP-18e column under a flow rate of 3 mL/min with only 25 pmol on column injection for each oligonucleotide. 50 mM of TEAA was used as mobile phase A and acetonitrile as mobile phase B with a gradient of 5% B ramping to 15% B in 10 minutes. The typical back pressure at 3 mL/min is 50 bar which is beneficial for high throughput assays.
Ion-Pairing Additive Concentration Test
In the qualitative and quantitative analysis of oligonucleotide impurities, ion-pair reversed-phase liquid chromatography has been the dominant technique. The ion-pairing reagents added in the mobile phase are typically several alkylammonium salts which are adsorbed on the column sorbent with the positive charges exposed to interact with the negatively charged oligonucleotides. Triethylammonium acetate (TEAA) is one of the commonly used ion-paring reagents in LC-UV analysis of oligonucleotides. Optimizing ion-pairing additive concentration is important to achieve efficient separation while minimizing the cost of additive consumption. In this work, optimization of TEAA concentration was conducted.
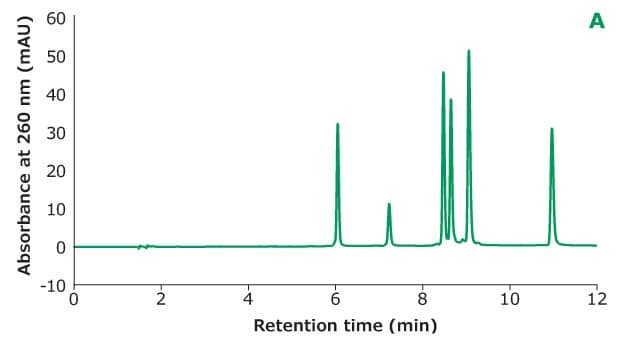
Figure 2A.Oligo Standard 6 separation on Chromolith® Performance RP-18e, 100 x 4.6 mm column with different TEAA concentration in mobile phase A: 50 mM TEAA. Resolution is calculated between every two adjacent peaks.
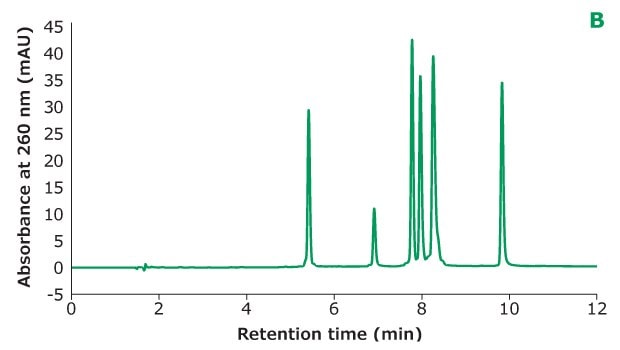
Figure 2B.Oligo Standard 6 separation on Chromolith® Performance RP-18e, 100 x 4.6 mm column with different TEAA concentration in mobile phase A: 20 mM TEAA. Resolution is calculated between every two adjacent peaks.
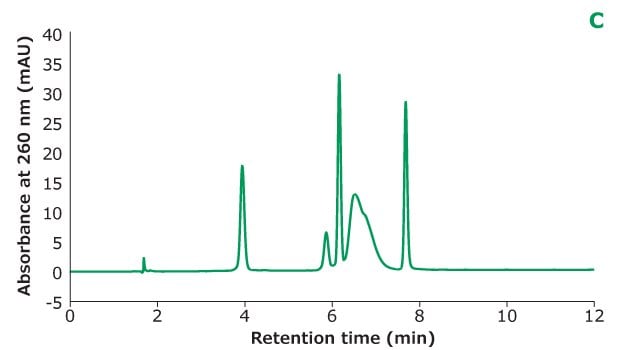
Figure 2C.Oligo Standard 6 separation on Chromolith® Performance RP-18e, 100 x 4.6 mm column with different TEAA concentration in mobile phase A: 5 mM TEAA. Resolution is calculated between every two adjacent peaks.
Figure 2 shows the different concentrations of TEAA tested in mobile phase A with acetonitrile as mobile phase B in the separation. Five microliters of Oligo Standard 6 sample were injected on a Chromolith® Performance RP-18e, 100 x 4.6 mm column at a flow rate of 1 mL/min with a gradient of 8% B to 15% B in 10 minutes for each test. With 50 mM of TEAA in mobile phase A, the oligonucleotides were well separated with the retention time for Oligo 1 to 6 at 6.051 min, 7.232 min, 8.476 min, 8.647 min, 9.058 min, and 10.964 min. When the TEAA concentration was lowered to 20 mM, Oligo 1 to 6 eluted in the same order but with less retention on the column. With the exception of Oligos 1 and 2, the resolution between each peak pair is seen to be lower as well. When TEAA concentration was further lowered to 5 mM, Oligos 4 and 5 were not separated which indicates the ion-pairing strength is not high enough to separate these two oligonucleotides. Comparing the peak heights of the six Oligos under the three different TEAA concentrations, 50 mM TEAA produced the highest peak height as shown in the table in Figure 2. Therefore, the ion-pairing additive concentration needs to be optimized based on the characteristics of the oligonucleotides.
Chromolith® HighResolution RP-18e Column, 2 mm I.D.
The Chromolith® HighResolution (HR) column possesses 1.15 µm macropores compared with 2 µm on the standard Chromolith® column. This modification results in higher separation efficiency and better peak shape. Although this creates higher back pressure, it is still less than half that of any particulate column of similar efficiency.4
Here, 3 µL of Oligo Standard 6 sample were injected onto the Chromolith® HighResolution RP-18e, 100 x 2.0 mm column at 0.4 mL/min with a gradient of 8% B to 15% B in 10 minutes. Figure 3 is an overlay of three injections showing consistent retention and response. 50 mM TEAA concentration was used as mobile phase A and acetonitrile was mobile phase B. Resolution between Oligo 4 and 5 is 4.936.

Figure 3.Oligo Standard 6 separation on Chromolith® HighResolution RP-18e, 100 x 2.0 mm column. Mobile phase A: 50 mM TEAA in water, Mobile phase B: acetonitrile; gradient: 8% B to 15% B in 10 minutes at a flow rate of 0.4 mL/min, column temp.: 40 oC, Injection: 3μl (15 pmol on column).
A shorter column of Chromolith® HighResolution RP-18e, 50 x 2 mm was compared with the same conditions with 5 µL of injection volume used in Figure 3. As shown in Figure 4, on a 50 x 2 mm column, all six oligonucleotides were eluted within 10 minutes with a resolution between Oligo 4 and 5 of 3.921. Thus, Chromolith® HR RP-18e column is capable of oligonucleotide analysis using LC-MS compatible flow rates.

Figure 4.Oligo Standard 6 separation on Chromolith® High Resolution RP-18e, 50 x 2.0 mm column. Mobile phase A: 50 mM TEAA in water, Mobile phase B: acetonitrile; gradient: 8% B to 15% B in 10 minutes at flow rate of 0.4 mL/min, column temp.: 40 oC, Injection: 5 μL (25 pmol on column).
Conclusion
In this application note, the separation of Oligo Standard 6, an internally created HPLC-UV system suitability mix, was demonstrated on standard Chromolith® and Chromolith® High-Resolution RP-18e columns. Flow rates up to 3 mL/min were evaluated on the standard Chromolith® with excellent separation of the six oligos indicating that it is ideal for high throughput assays. The effects of ion-pairing reagent, TEAA, on Oligo Standard 6 separation was discussed to provide optimization guidance in oligo analysis. Chromolith® High-Resolution RP-18e columns were evaluated with a typical LC-MS flow rate demonstrating this column is suitable for oligonucleotide analysis by mass spectrometry. In addition, the polymeric column housing can be used as part of a metal-free, or bio-inert HPLC system.
Acknowledgment
The authors would like to thank Pierre Potier for providing the Standard Six oligonucleotide mix and the technical support.
References
To continue reading please sign in or create an account.
Don't Have An Account?