Recommended Products
biological source
egg yolk
form
powder
storage temp.
−20°C
Looking for similar products? Visit Product Comparison Guide
General description
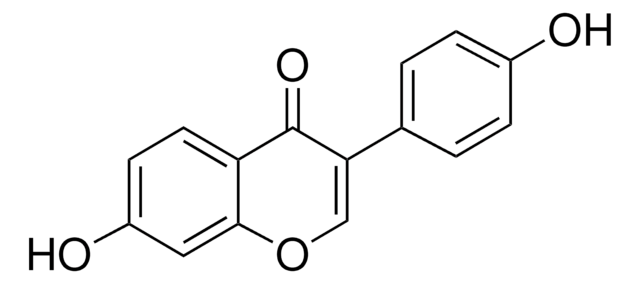
Phosvitin from egg yolk is a polyanionic phosphoserine rich protein. This phosphoglycoprotein comprises α-helices and β-sheets structural elements, which are poorly organized due to the presence of phosphoserine. It represents about 11% of yolk proteins and is hydrophilic.
Application
Phosvitin from egg yolk has been used:
- as a control protein for immobilization on-chip for deep purple protein staining methods for monitoring post-translation modifications
- as a substrate for casein kinase Iε in in vitro casein kinase assay
- as a standard in gel filtration chromatography (GFC) and sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) for quantification of phosvitin from egg extracts
- for immobilization onto Layer-by-Layer (LbL) for Fourier transform infrared spectroscopy-attenuated total reflectance (FTIR-ATR) studies
Biochem/physiol Actions
Phosvitin fed to rats by adding egg yolk to their food resulted in decreased absorption of magnesium, iron and calcium but not of phosphorus.
Phosvitin from egg yolk is a calcium chelator and binds to iron present in the yolk. The metal-binding functionality is exploited in mineral-binding bioactive peptide production. It is a nutraceutical and has potential for phosphopeptides production.
Other Notes
A phosophoprotein containing 8-10% phosphorus. Molar N/P ratio approx. 2.7.
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Himali Samaraweera et al.
Journal of food science, 76(7), R143-R150 (2011-08-03)
Phosphopeptides are among the most interesting biomolecules with characteristic molecular structure and functions. They usually contain clusters of phosphoserines, which can effectively bind calcium and iron, and inhibit formation of insoluble calcium phosphates or iron complexes. Therefore, phosphopeptides can increase
Shuichi Ito et al.
Journal of biomedical materials research. Part A, 100(10), 2760-2765 (2012-05-25)
The aim of this study is to evaluate the mineralizing potential of acidic monomers and their calcium salts for mineralization, using an in vitro mineral induction model. Phosvitin (PV) was used as a model phosphoprotein in this study. PV was
Shuichi Ito et al.
Journal of dentistry, 39(1), 72-79 (2010-10-26)
The aim of this study was to evaluate the mineralizing potential of ions released from surface pre-reacted glass-ionomer (S-PRG) fillers on mineral induction by phosphoprotein in vitro. Phosvitin was used as a model of dentin phosphoprotein in this study. Phosvitin
S-I Ishikawa et al.
Journal of food science, 72(6), S412-S419 (2007-11-13)
Egg yolk decreases the absorption of iron. The effects of egg yolk protein and egg yolk phosvitin on the absorption of calcium, magnesium, and iron were investigated by in vivo studies. Male Wistar rats were fed purified diets containing casein
Daniel S Schabacker et al.
Analytical biochemistry, 359(1), 84-93 (2006-10-13)
Methods to assess the quality and performance of protein microarrays fabricated from undefined protein content are required to elucidate slide-to-slide variability and interpolate resulting signal intensity values after an interaction assay. We therefore developed several simple total- and posttranslational modification-specific
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service