36323
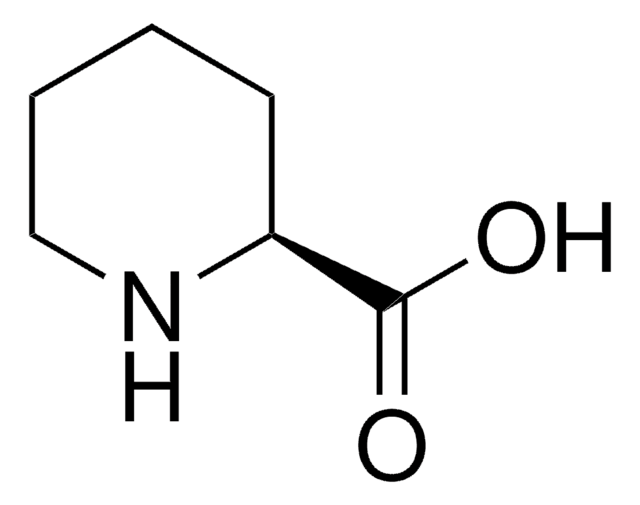
(S)-6-Oxo-2-piperidinecarboxylic acid
≥95.0% (HPLC)
Synonym(s):
6-Oxo-L-pipecolic acid, (S)-2-Piperidone-6-carboxylic acid, L-Pyrohomoglutamic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C6H9NO3
CAS Number:
Molecular Weight:
143.14
MDL number:
UNSPSC Code:
12352005
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
≥95.0% (HPLC)
impurities
≤5.0% water
SMILES string
OC(=O)[C@@H]1CCCC(=O)N1
InChI
1S/C6H9NO3/c8-5-3-1-2-4(7-5)6(9)10/h4H,1-3H2,(H,7,8)(H,9,10)/t4-/m0/s1
InChI key
FZXCPFJMYOQZCA-BYPYZUCNSA-N
Related Categories
Application
(S)-6-Oxo-2-piperidinecarboxylic acid can be used as a reactant to synthesize:
- Functionalized β-lactam N-heterocycles via carboxymethylproline synthase catalyzed cyclization reactions. Pro-(S)-C5 branched [4.3.1] aza-amide bicycles as potential inhibitors for FK506-binding proteins.
- Ethyl (S)-2-(6-oxopiperidin-2-yl)acetate, which is reduced using LiBH4 to produce optically pure hydroxymethyl lactams.
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 2
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Preparation of optically pure ω-hydroxymethyl lactams
Huang S-B, et al.
Synthetic Communications, 19(20), 3485-3496 (1989)
Rational Design and Asymmetric Synthesis of Potent and Neurotrophic Ligands for FK506-Binding Proteins (FKBPs)
Pomplun S, et al.
Angewandte Chemie (International ed. in English), 54(1), 345-348 (2015)
Carboxymethylproline synthase catalysed syntheses of functionalised N-heterocycles
Hamed RB, et al.
Chemical Communications (Cambridge, England), 46(9), 1413-1415 (2010)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service