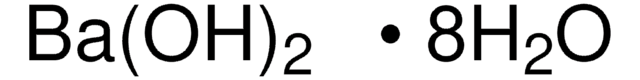All Photos(1)
About This Item
Linear Formula:
BaCO3
CAS Number:
Molecular Weight:
197.34
Beilstein/REAXYS Number:
7045119
EC Number:
MDL number:
UNSPSC Code:
12352302
PubChem Substance ID:
Recommended Products
grade
SAJ first grade
assay
≥98.0%
form
powder
availability
available only in Japan
pH
6.8 (37 °C, 3.67 g/L)
SMILES string
[Ba++].[O-]C([O-])=O
InChI
1S/CH2O3.Ba/c2-1(3)4;/h(H2,2,3,4);/q;+2/p-2
InChI key
AYJRCSIUFZENHW-UHFFFAOYSA-L
Looking for similar products? Visit Product Comparison Guide
signalword
Warning
hcodes
Hazard Classifications
Acute Tox. 4 Oral
Storage Class
13 - Non Combustible Solids
wgk_germany
WGK 1
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Chengdu Qi et al.
Chemosphere, 260, 127681-127681 (2020-08-08)
In this work, magnetic separably barium ferrite nanomaterial (BaFeO) was synthesized via citrate acid assisted sol-gel combustion method. Subsequently, X-ray diffraction (XRD), scanning electron microscopy-energy dispersion spectroscopy (SEM-EDS), transmission electron microscopy (TEM) and vibrating sample magnetometer (VSM) were applied for
Miyako Akiyoshi et al.
Journal of hazardous materials, 132(2-3), 141-147 (2005-12-14)
Iron picrate (FePic) was synthesized under conditions similar to those that result in the natural deterioration of chemical weapons. Its thermal hazard was investigated by comparing it with iron picrate obtained by the chemical synthesis method (FePic(Ba)). FePic has eight
Matthias Kellermeier et al.
Journal of colloid and interface science, 380(1), 1-7 (2012-06-05)
Under certain conditions, precipitation of barium carbonate in alkaline silica-rich environments affords unusual polycrystalline aggregates exhibiting curved shapes and hierarchical structuring, very much reminiscent of biogenic mineral tissues. The formation of these so-called silica biomorphs is thought to rely on
Mallikarjuna N Nadagouda et al.
Journal of hazardous materials, 188(1-3), 19-25 (2011-02-01)
BaCO(3) dispersed PVC composites were prepared through a polymer re-precipitation method. The composites were tested for sulfate removal using rapid small scale column test (RSSCT) and found to significantly reduce sulfate concentration. The method was extended to synthesize barium carbonate-loaded
D Ekeberg et al.
Carbohydrate research, 335(2), 141-146 (2001-09-25)
A simple, but low-yielding method for the synthesis of 3-hexuloses has been elaborated. Oxidation of 1,2:5,6-di-O-isopropylidenehexitols with bromine in the presence of barium carbonate, followed by mild-acid hydrolysis of the oxidation products gave the free hexuloses. Oxidation occurred at only
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service