808423
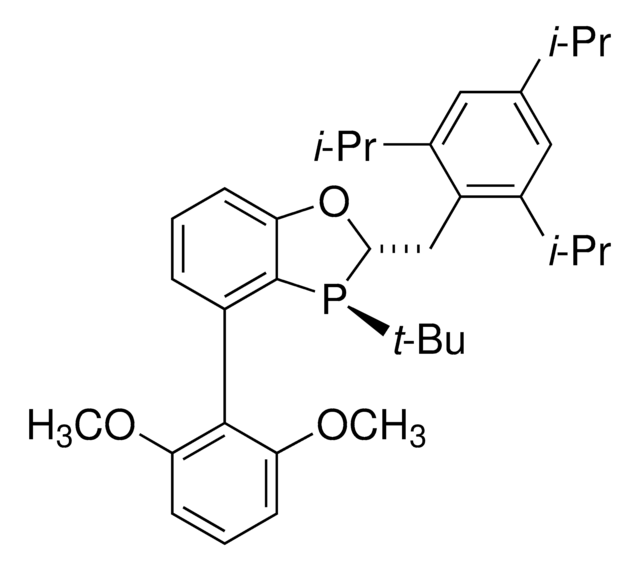
HandaPhos
toluene solution (14 mg HandaPhos per 1 mL of toluene)
About This Item
Recommended Products
form
liquid
reaction suitability
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reaction type: Heck Reaction
reaction type: Hiyama Coupling
reaction type: Negishi Coupling
reaction type: Sonogashira Coupling
reaction type: Stille Coupling
reaction type: Suzuki-Miyaura Coupling
reagent type: ligand
transition temp
flash point 42.0 °F
functional group
phosphine
storage temp.
2-8°C
SMILES string
COC(C=CC=C1OC)=[C@]1[C@@]2=C([P@](C(C)(C)C)[C@@H](CC3=C(C(C)C)C=C(C(C)C)C=C3C(C)C)O4)C4=CC=C2
Application
Other Notes
From milligrams to kilograms: synthetic chemistry following nature′s lead
related product
signalword
Danger
Hazard Classifications
Aquatic Chronic 3 - Asp. Tox. 1 - Flam. Liq. 2 - Repr. 2 - Skin Irrit. 2 - STOT RE 2 - STOT SE 3
target_organs
Central nervous system
Storage Class
3 - Flammable liquids
wgk_germany
WGK 3
flash_point_f
41.0 °F
flash_point_c
5 °C
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Related Content
Prof. Bruce Lipshutz and co-workers have developed designer surfactants to allow several classes of transformations (e.g. Suzuki-Miyaura, Olefin Metathesis, 1,4-Addition to Enones, etc.) to be performed in water.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service