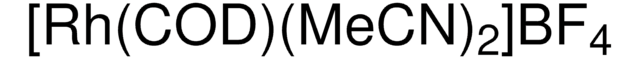683175
Chiralyst P374
Umicore
Synonym(s):
Bis(norbornadiene)rhodium(I) tetrafluoroborate, Bis(bicyclo[2.2.1]hepta-2,5-diene)rhodium tetrafluoroborate, Bis(norbornadiene)(tetrafluoroborato)rhodium, Rh(nbd)2BF4, Rh(nbd)2BF4
About This Item
Recommended Products
form
solid
reaction suitability
core: rhodium
reagent type: catalyst
mp
136.9-139.9 °C
SMILES string
[Rh+].F[B-](F)(F)F.C1C2C=CC1C=C2.C3C4C=CC3C=C4
InChI
1S/2C7H8.BF4.Rh/c2*1-2-7-4-3-6(1)5-7;2-1(3,4)5;/h2*1-4,6-7H,5H2;;/q;;-1;+1/t2*6-,7+;;
InChI key
HAYDJWBQWOEERB-JAGGYEKFSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
- Lactones via the 1,4-addition of arylboronic acids to butenolide.
- Chiral β-aryl-substituted ketones and esters.
- β-aryloxycarboxylic esters from β-aryloxy-α,γ-unsaturated esters.
Legal Information
signalword
Danger
hcodes
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B
Storage Class
8A - Combustible corrosive hazardous materials
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Precatalysts for asymmetric catalysis offer batch-to-batch consistency in a range of catalytic reactions, supporting diverse research needs.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service![(R)-3,3′-Bis[3,5-bis(trifluoromethyl)phenyl]-1,1′-binaphthyl-2,2′-diyl hydrogenphosphate 95%](/deepweb/assets/sigmaaldrich/product/structures/270/636/14dc9413-bcb4-478c-8e4d-3605317c13a5/640/14dc9413-bcb4-478c-8e4d-3605317c13a5.png)






![Bis(1,5-cyclooctadiene)rhodium(I) tetrakis[bis(3,5-trifluoromethyl)phenyl]borate](/deepweb/assets/sigmaaldrich/product/structures/275/399/3f196135-3b90-4f6f-95b0-512766f1f5e1/640/3f196135-3b90-4f6f-95b0-512766f1f5e1.png)
![(S,R,R)-(+)-(3,5-Dioxa-4-phosphacyclohepta[2,1-a:3,4-a′]dinaphthalen-4-yl)bis(1-phenylethyl)amine 95% (HPLC)](/deepweb/assets/sigmaaldrich/product/structures/366/790/7555ef31-5d0b-45c9-ad40-5dfd0fe85125/640/7555ef31-5d0b-45c9-ad40-5dfd0fe85125.png)