All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C10H12O4
CAS Number:
Molecular Weight:
196.20
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
97%
form
solid
mp
80-84 °C (lit.)
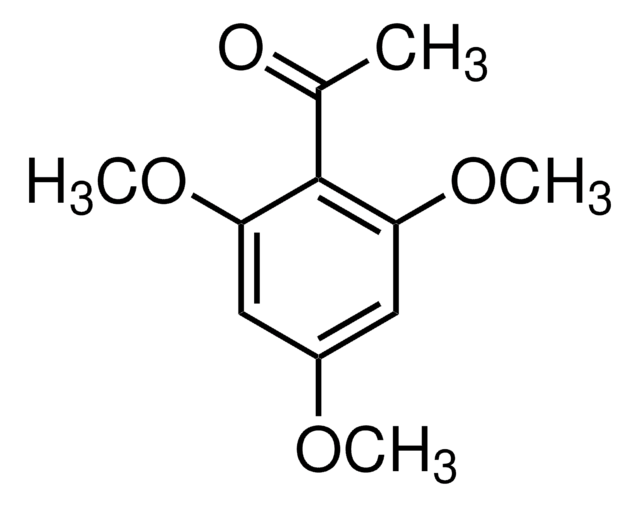
SMILES string
COc1cc(O)c(C(C)=O)c(OC)c1
InChI
1S/C10H12O4/c1-6(11)10-8(12)4-7(13-2)5-9(10)14-3/h4-5,12H,1-3H3
InChI key
FBUBVLUPUDBFME-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Fungicide and fungiostatic effects of xanthoxyline.
V Cechinel Filho et al.
Journal of ethnopharmacology, 53(3), 171-173 (1996-09-01)
V Cechinel Filho et al.
Journal of pharmaceutical sciences, 84(4), 473-475 (1995-04-01)
The antispasmodic activity of several xanthoxyline derivatives against acetylcholine-induced contraction of the guinea pig ileum was evaluated in vitro. The acetophenones with two methoxyl groups, mainly in the 3,4 positions, exhibited potent antispasmodic activity. Modification of the hydroxyl group in
Paula Boeck et al.
Archiv der Pharmazie, 338(2-3), 87-95 (2005-03-31)
Chalcones and chalcone-like compounds, most of them new ones, prepared by base-catalyzed condensation of appropriate aldehydes and xanthoxyline, were tested for antifungal properties against a panel of yeasts, hialohyphomycetes as well as dermatophytes with the agar dilution assay. Results indicate
C Tringali et al.
Fitoterapia, 72(5), 538-543 (2001-06-29)
Analysis of the polar fractions of an EtOH extract obtained from the bark of the African medicinal plant Fagara macrophylla led to the isolation and identification of the alkaloids oblongine (6), tembetarine (7) and magnoflorine (8) and the flavonoid hesperidin
Rodrigo dos Santos et al.
Archiv der Pharmazie, 339(5), 227-237 (2006-03-31)
Semi-empirical molecular orbital calculations at AM1 level were done with the aim to investigate the structure-activity relationships of antispasmodic activities of ten 2-(X-benzyloxy)-4,6-dimethoxyacetophenones with X = H, 4'-F, 4'-NO2, 4'-CH3, 4'-Cl, 3',4'-(CH3)2, 4'-OCH3, 4'-Br, 4'-OCH2C6H5, and 4'-C(CH3)3, against acetylcholine-induced contraction
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service