All Photos(1)
About This Item
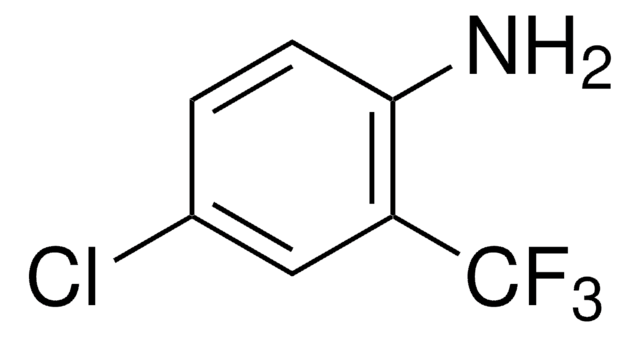
Linear Formula:
CH3OC6H3(I)CO2CH3
CAS Number:
Molecular Weight:
292.07
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
98%
mp
92-95 °C (lit.)
SMILES string
COC(=O)c1ccc(OC)c(I)c1
InChI
1S/C9H9IO3/c1-12-8-4-3-6(5-7(8)10)9(11)13-2/h3-5H,1-2H3
InChI key
GHNGBFHLUOJHKP-UHFFFAOYSA-N
Application
Methyl 3-iodo-4-methoxybenzoate (1,3-Dimethyl-1H-pyrazol-5-amine) may be used in the preparation of:
- methyl 3-[3-(N,N-dimethylamino)prop-1-ynyl]-4-methoxybenzoate
- 3,4,5,2′-tetramethoxybiphenyl
- 5-propyl-3-ol-1,3-dimethyl-1H-pyrazolo[3,4-b]pyridine
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Aucuparin and Methoxyaucuparin, Two Phenolic Biphenyl.
Erdtman H, et al.
Acta Chemica Scandinavica, 17(4), 1151-1156 (1963)
M Lamothe et al.
Journal of medicinal chemistry, 40(22), 3542-3550 (1997-11-14)
The synthesis and binding affinity at cloned h5-HT1D, h5-HT1D, and h5-HT1A receptors of 3-[3-(N,N-dimethylamino)propyl]-4-hydroxy- N-[4-(pyridin-4-yl)phenyl]benzamide (2, GR-55562) and four O-methylated analogs are described. The functional activity of these compounds was determined at the h5-HT1B receptor using a [35S]GTP gamma S
Sandip B Bharate et al.
Bioorganic & medicinal chemistry, 16(15), 7167-7176 (2008-07-16)
In the present article, we have synthesized three different series of pyrazolo[3,4-b]pyridines and their structural analogues using novel synthetic strategy involving one-pot condensation of 5,6-dihydro-4H-pyran-3-carbaldehyde/2-formyl-3,4,6-tri-O-methyl-D-glucal/chromone-3-carbaldehyde with heteroaromatic amines. All synthesized compounds were evaluated for their anti-inflammatory activity against TNF-alpha and
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)
![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II), complex with dichloromethane](/deepweb/assets/sigmaaldrich/product/structures/825/986/4317978b-1256-4c82-ab74-6a6a3ef948b1/640/4317978b-1256-4c82-ab74-6a6a3ef948b1.png)
![5-Chloro-3-methylbenzo[b]thiophene-2-sulfonyl chloride](/deepweb/assets/sigmaaldrich/product/structures/545/778/7ff21d5d-2b44-4e6f-a52a-47bc016d1383/640/7ff21d5d-2b44-4e6f-a52a-47bc016d1383.png)

![2,3-dihydrobenzo[b]furan-5-carboxylic acid AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/130/501/0c91be6e-e783-4788-96e2-fec35497ea05/640/0c91be6e-e783-4788-96e2-fec35497ea05.png)