513997
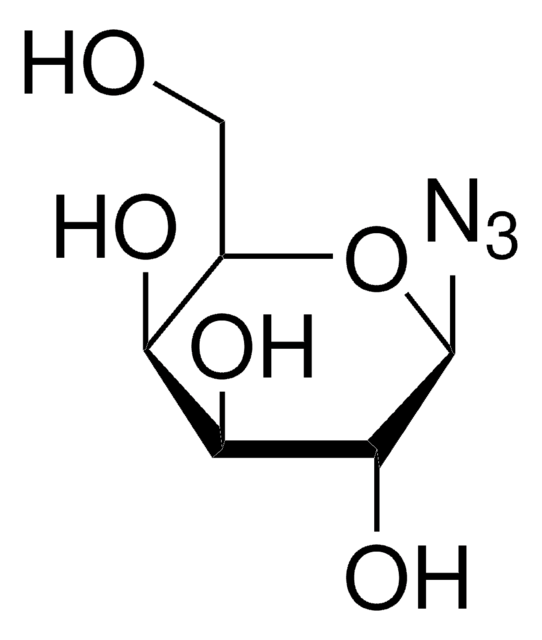
1-Azido-1-deoxy-β-D-glucopyranoside tetraacetate
Synonym(s):
NSC 272456
About This Item
Recommended Products
form
solid
optical activity
[α]/D -29°, c = 1% in H2O
[α]/D -30°, c = 1% in chloroform
reaction suitability
reaction type: click chemistry
mp
127-131 °C (lit.)
SMILES string
CC(=O)OC[C@H]1O[C@@H](N=[N+]=[N-])[C@H](OC(C)=O)[C@@H](OC(C)=O)[C@H]1OC(C)=O
InChI
1S/C14H19N3O9/c1-6(18)22-5-10-11(23-7(2)19)12(24-8(3)20)13(25-9(4)21)14(26-10)16-17-15/h10-14H,5H2,1-4H3/t10-,11+,12+,13-,14-/m1/s1
InChI key
NHNYHKRWHCWHAJ-MBJXGIAVSA-N
Application
- 1,2,3-Triazole-boron dipyrromethenes (BODIPYs) containing glucose groups via Cu(I)-catalyzed azide–alkyne ″click″ cycloaddition reaction conditions.
- 1-(β-D-glycosyl)-5-benzenesulfonamide-1,2,3-triazole derivatives by ruthenium-catalyzed azide-alkyne cycloaddition reactions.
- 2,3,4,6-Tetra-O-acetyl-β-D-glucopyranosylamine by palladium catalyzed hydrogenation reaction.
- Glycoside annulated dihydropyrimidinone derivatives by one-pot five-component condensation reaction with tert-butyl β-ketoester, arylaldehyde, urea and propargyl alcohol.
- Synthesis of Protein Tyrosine Phosphatase 1B inhibitor
- Synthesis of glycoconjugate carbonic anhydrase inhibitors by ruthenium-catalyzed azide-alkyne 1,3-dipolar cycloaddition
- Synthesis of variously coupled conjugates of D-glucose via click chemistry for inhibition of glycogen phosphorylase
- Hydrogenation reactions
- Preparation of posttranslationally modified peptides efficiently mimicking neoantigens in relation to autoimmune disease
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service