465089
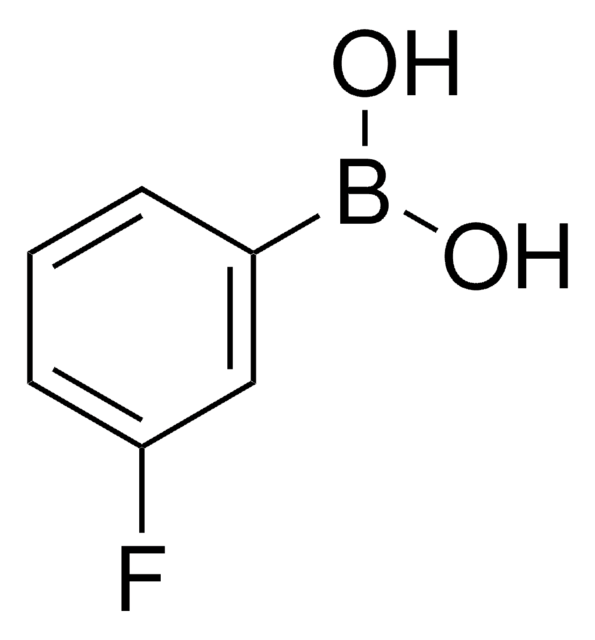
3,4-Difluorophenylboronic acid
≥95%
Synonym(s):
3,4-Difluorobenzeneboronic acid
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
F2C6H3B(OH)2
CAS Number:
Molecular Weight:
157.91
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
≥95%
mp
305-310 °C (lit.)
SMILES string
OB(O)c1ccc(F)c(F)c1
InChI
1S/C6H5BF2O2/c8-5-2-1-4(7(10)11)3-6(5)9/h1-3,10-11H
InChI key
RMGYQBHKEWWTOY-UHFFFAOYSA-N
Application
3,4-Difluorophenylboronic acid can be used as a reactant to prepare:
- Fluorinated biaryl derivatives via Suzuki cross-coupling reaction with aryl and heteroaryl halides.
- Flurodiarylmethanols by reacting with aryl aldehydes using Ni catalyst.
- Conjugated fluorodiazaborinines by treating with diamines via intermolecular dehydration reaction for the detection of explosives.
Reactant involved in:
- Suzuki cross-coupling reactions with aryl and heteroaryl halides
- Oxo directing Liebeskind-Srogl cross-coupling reactions with gem-dihaloolefin-type α-oxo ketene dithioacetals
- Substitution reactions with enyne acetates and carbonates
Other Notes
Contains varying amounts of anhydride
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Green synthesis of fluorinated biaryl derivatives via thermoregulated ligand/palladium-catalyzed Suzuki reaction
L Ning, et al.
Journal of Organometallic Chemistry, 696(13), 2641-2647 (2011)
NBN-Doped Conjugated Polycyclic Aromatic Hydrocarbons as an AIEgen Class for Extremely Sensitive Detection of Explosives
Wan W-M, et al.
Angewandte Chemie (International Edition in English), 57(47), 15510-15516 (2018)
Nickel salt-catalyzed addition reaction of arylboronic acids to aromatic aldehydes
Zhou L, et al.
Tetrahedron Letters, 50(4), 406-408 (2009)
Mingyan Jia et al.
Food chemistry, 335, 127566-127566 (2020-08-04)
In this work, we developed an optical colorimetric sensor array for the discrimination of Chinese teas. The sensor array was carefully designed based on tea polyphenol induced indicators displacement assay (IDA), using phenylboronic acids with different substituents as the receptors
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service



![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II), complex with dichloromethane](/deepweb/assets/sigmaaldrich/product/structures/825/986/4317978b-1256-4c82-ab74-6a6a3ef948b1/640/4317978b-1256-4c82-ab74-6a6a3ef948b1.png)