All Photos(1)
About This Item
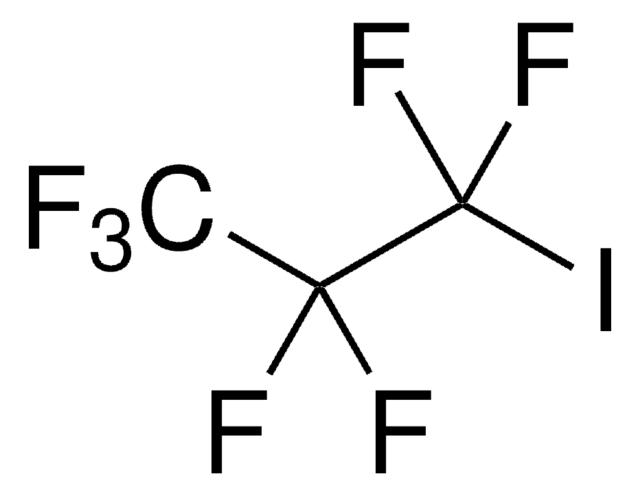
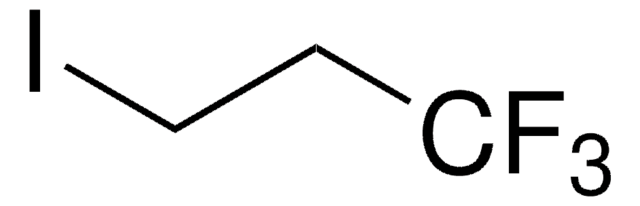
Linear Formula:
BrCH2CH2CF3
CAS Number:
Molecular Weight:
176.96
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
98%
refractive index
n20/D 1.363 (lit.)
bp
63.5 °C (lit.)
density
1.662 g/mL at 25 °C (lit.)
SMILES string
FC(F)(F)CCBr
General description
3-Bromo-1,1,1-trifluoropropane is a halogenated hydrocarbon.
Application
3-Bromo-1,1,1-trifluoropropane may be used for the synthesis of 3-(benzyloxy)-N-3,3,3-trifluoropropyl-16,17-seco-estra-1,3,5(10)-triene-16,17-imide and [N-hydroxy-N-(1-trifluoromethylethenyl)]amido
diethylphosphate.
diethylphosphate.
Please view www.aldrich.com/epaods regarding the EPA′s request for application information of Ozone Depleting Substances
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Ozone 1 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
10 - Combustible liquids
wgk_germany
WGK 3
flash_point_f
235.4 °F - closed cup
flash_point_c
113 °C - closed cup
ppe
Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Phosphorus substituted hydroxylamine and hydroxamic acid derivatives: synthesis and reactivity.
Alonso C, et al.
ARKIVOC (Gainesville, FL, United States), 221-253 (2011)
Y Tanaka et al.
Archives of biochemistry and biophysics, 263(1), 178-190 (1988-05-15)
Leukotriene B4 is rapidly metabolized through omega-oxidation, preventing its detection when it is produced under certain biological conditions. To investigate leukotriene B4 production in various physiological conditions, analogs of arachidonic acid which are converted to metabolically stable analogs of leukotriene
L W Lawrence Woo et al.
Molecular cancer therapeutics, 7(8), 2435-2444 (2008-08-30)
An improved steroid sulfatase inhibitor was prepared by replacing the N-propyl group of the second-generation steroid-like inhibitor (2) with a N-3,3,3-trifluoropropyl group to give (10). This compound is 5-fold more potent in vitro, completely inhibits rat liver steroid sulfatase activity
Tetrahedron Letters, 37, 5557-5557 (1996)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service