All Photos(1)
About This Item
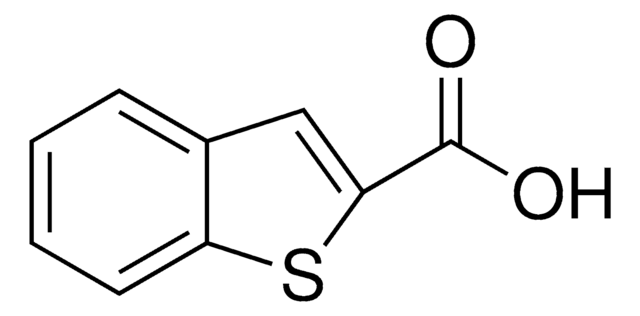
Linear Formula:
CH3OC6H3(NO2)CO2H
CAS Number:
Molecular Weight:
197.14
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
98%
mp
192-194 °C (lit.)
SMILES string
COc1ccc(cc1[N+]([O-])=O)C(O)=O
InChI
1S/C8H7NO5/c1-14-7-3-2-5(8(10)11)4-6(7)9(12)13/h2-4H,1H3,(H,10,11)
InChI key
ANXBDAFDZSXOPQ-UHFFFAOYSA-N
Related Categories
Application
4-Methoxy-3-nitrobenzoic acid was used in the synthesis of:
- BIPHEP-1-OMe [BIHEP= (2,2′-bis(diphenylphosphino)biphenyl)], precatalyst for reductive coupling of butadiene to aldehydes
- aniline mustard analogues, having potential anti-tumor activity
signalword
Warning
hcodes
Hazard Classifications
Acute Tox. 4 Oral
Storage Class
11 - Combustible Solids
wgk_germany
WGK 1
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Jason R Zbieg et al.
Advanced synthesis & catalysis, 352(14-15), 2416-2420 (2010-12-18)
Exposure of alcohols 1a-1i to butadiene in the presence of a cyclometallated iridium catalyzed derived from allyl acetate, 4-methoxy-3-nitrobenzoic acid and BIPHEP (2,2'-bis(diphenylphosphino)biphenyl) results in hydrogen transfer to generate aldehyde-allyliridium pairs, which engage in C-C coupling to form products of
B G Choi et al.
Archives of pharmacal research, 21(2), 157-163 (1999-01-06)
Several aniline mustard analogues were obtained by introducing N,N-bis(2-chloroethyl)amino moiety to phenyl ring of A10 analogues in order to increase reactivity of A10 analogs and selectivity into DNA. The in vitro antitumor activity of synthesized compounds was evaluated using five
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service