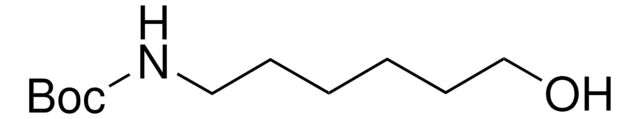
17356
3-(Boc-amino)propyl bromide
≥96.0% (GC)
Synonym(s):
tert-Butyl N-(3-bromopropyl)carbamate
Select a Size
Select a Size
About This Item
Recommended Products
Quality Level
assay
≥96.0% (GC)
reaction suitability
reagent type: cross-linking reagent
mp
37-39 °C
functional group
Boc
amine
bromo
storage temp.
2-8°C
SMILES string
BrCCCNC(OC(C)(C)C)=O
InChI
1S/C8H16BrNO2/c1-8(2,3)12-7(11)10-6-4-5-9/h4-6H2,1-3H3,(H,10,11)
InChI key
IOKGWQZQCNXXLD-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
- Benzydamine analogs to be used as activators for soluble guanylate cyclase.[1]
- N-substituted chromenotriazolopyrimidine, human murine double minute 2 (MDM2) inhibitor.[2]
- Protected amines from piperidine derivatives to be further used for synthesis of sulfonamide series.[3]
It can also be used for the post-polymerization quaternization of polymers to synthesize functional cationic polymers and antimicrobial agents.[4]
signalword
Warning
hcodes
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Active Filters
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service