122408
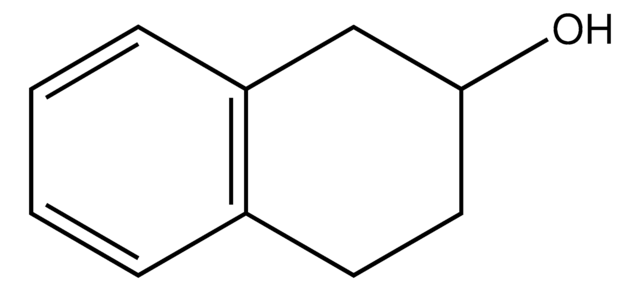
1,2,3,4-Tetrahydro-1-naphthol
97%
Synonym(s):
α-Tetralol
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C10H12O
CAS Number:
Molecular Weight:
148.20
Beilstein/REAXYS Number:
2046227
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
97%
form
solid
refractive index
n20/D 1.564 (lit.)
bp
102-104 °C/2 mmHg (lit.)
density
1.09 g/mL at 25 °C (lit.)
SMILES string
OC1CCCc2ccccc12
InChI
1S/C10H12O/c11-10-7-3-5-8-4-1-2-6-9(8)10/h1-2,4,6,10-11H,3,5,7H2
InChI key
JAAJQSRLGAYGKZ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
(R)-(-)-enantiomer of 1,2,3,4-Tetrahydro-1-naphthol is a substrate for aryl sulfotransferase (AST) IV enzyme and (S)-(+)-1,2,3,4-tetrahydro-1-naphthol is a competitive inhibitor of AST IV-catalyzed sulfation of 1-naphthalenemethanol. It is the major urinary metabolite of tetralin.
Application
1,2,3,4-Tetrahydro-1-naphthol was used as chiral probe to examine the role of three aromatic residues in enzyme-substrate interactions at the sulfuryl acceptor binding site of aryl sulfotransferase IV enzyme.
signalword
Warning
hcodes
pcodes
Hazard Classifications
Acute Tox. 4 Oral
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
ppe
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Jonathan J Sheng et al.
Drug metabolism and disposition: the biological fate of chemicals, 32(5), 559-565 (2004-04-22)
Aryl sulfotransferase (AST) IV (also named tyrosine-ester sulfotransferase and ST1A1) is a major phenol sulfotransferase in the rat, and it catalyzes the sulfation of many drugs, carcinogens, and other xenobiotics that contain phenol, benzylic alcohol, N-hydroxy arylamine, and oxime functional
Metabolism of tetralin and toxicity of Cuprex in man.
D E Drayer et al.
Drug metabolism and disposition: the biological fate of chemicals, 1(3), 577-579 (1973-05-01)
Vyas Sharma et al.
Journal of medicinal chemistry, 45(25), 5514-5522 (2002-12-03)
Comparative Molecular Field Analysis (CoMFA) methods were used to produce a 3D-QSAR model that correlated the catalytic efficiency of rat hepatic aryl sulfotransferase (AST) IV, expressed as log(k(cat)/K(m)), with the molecular structures of its substrates. A total of 35 substrate
W F Leebaw et al.
The Journal of clinical endocrinology and metabolism, 47(3), 480-487 (1978-09-01)
Although the role of the neurotransmitter, dopamine (DA), in the regulation of PRL has been well documented, controversy exists regarding its participation in the regulation of the other pituitary hormones. Consequently, we infused DA into six healthy male subjects (ages
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service