QC Workflow Products for Medical Device Manufacturing
Leverage our global resources and extensive network to optimize your QC processes.
Analytical and microbial testing is crucial to ensure the quality and safety of medical devices. But in-process testing and quality control are more than just following procedures. Accurate and reliable results depend on choosing the right materials for calibration and validation of instruments, development and validation of testing methods, and the reproducible day-to-day testing. Well-defined materials and specifications ensure you choose the right materials for your analyses.
We are committed to providing exceptional support and resources to ensure a seamless and reliable quality control (QC) workflow experience and to deliver the best products to improve health outcomes.
Explore our comprehensive range of products and services for quality control applications:
Fill out a short form to request a consultation with a specialist.

From HPLC to GC, our high-quality products ensure accurate results across diverse applications. Explore our complete range and elevate your analytical processes.

Find the perfect reagents for your needs. From wet chemistry to spectroscopy, our high-purity offerings are tailored for diverse analytical applications.
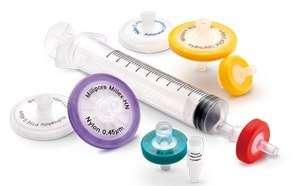
From SPE to air monitoring, ensure precision and refine your quality control with our comprehensive range products for diverse sample prep needs.

Discover a wide selection of labware and supplies from trusted brands to support your diverse research needs.

Simplify your microbial limit test workflow from filtration to culturing and detection. Choose accuracy for quality control with our complete portfolio.
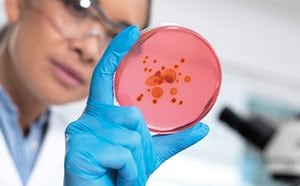
Streamline microbiological QC with specific proteins, biochemical activities, and gene sequences. Discover user-friendly solutions for accurate results.
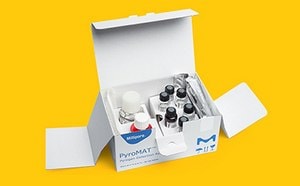
Adhere to regulatory standards and uphold high-quality products with our comprehensive portfolio for dependable testing.

Improve your quality control with more 20,000 products for various industries. Our standards are double accredited to ISO 17034 and ISO/IEC 17025.

Ensure accurate sterility testing and rely on our extensive quality control solutions for regulatory compliance and confidence in your results.
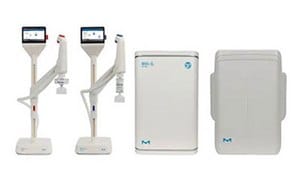
Innovative systems offer consistent quality, sustainability, and compliance. Find the perfect solution for your lab's needs, consult with experts, or explore our selection guide.
To explore more, visit our Medical Device Manufacturing home page for a comprehensive overview of products and services.
Our Products and Services Support The Following Areas
Explore each workflow step to learn more and find the right products & services to meet your needs.
Step 1: R&D
Step 2: Manufacturing
Step 3: QC

Medical Device Manufacturing Contact Form
Fill out this form to connect with a MilliporeSigma representative—an extensively trained Medical Device Manufacturing Specialist—who is your single point of contact for securing a steady supply of the right quality products.
Fields marked with an * are required.
To continue reading please sign in or create an account.
Don't Have An Account?
