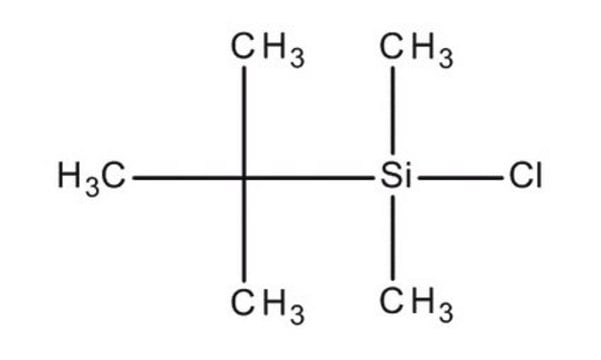
33092-U
tert-Butyldimethylsilylimidazole solution
TBDMSIM in DMF, pkg of 10 × 1 mL
Synonym(s):
1-(tert-Butyldimethylsilyl)imidazole solution
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C9H18N2Si
CAS Number:
Molecular Weight:
182.34
UNSPSC Code:
12000000
Recommended Products
description
Silylation reagent
packaging
pkg of 10 × 1 mL
concentration
TBDMSIM in DMF
InChI
1S/C9H18N2Si/c1-9(2,3)12(4,5)11-7-6-10-8-11/h6-8H,1-5H3
InChI key
VUENSYJCBOSTCS-UHFFFAOYSA-N
General description
tert-Butyldimethylsilylimidazole solution is a derivatizing reagent. It is most effective for silylating multiwall carbon nanotubes (MWNT).
Application
It was used to convert oxysterols to tert-butyldimethylsilyl (TBDMSi) ethers for 18O enrichment during oxysterols determination in plasma and liver, using gas/liquid chromatography-mass spectrometry analysis.
related product
Product No.
Description
Pricing
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Inhalation - Aquatic Chronic 3 - Eye Dam. 1 - Flam. Liq. 2 - Repr. 1B - Skin Corr. 1A
Storage Class Code
3 - Flammable liquids
WGK
WGK 2
Flash Point(F)
71.6 °F - closed cup
Flash Point(C)
22 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
O Breuer et al.
The Journal of biological chemistry, 270(35), 20278-20284 (1995-09-01)
Cholesterol oxidation products (oxysterols) have been detected in many different tissues, often at concentrations 10(3) to 10(4) times lower than cholesterol. This constitutes a considerable risk of quantitation errors, since even a minor oxidation of cholesterol during sample processing would
Yury Gogotsi
Nanotubes and Nanofibers, 46-46 (2006)
E.J.Corey and A. Venkateswarlu
Journal of the American Chemical Society, 94, 6190-6190 (1972)
D C Landrum et al.
Journal of chromatography, 483, 21-32 (1989-12-08)
The use of gas-liquid chromatography and mass spectrometry with derivatizing agents that give stable derivatives and consistent fragmentation patterns allows for accurate identification of a variety of compounds. In this study either N-methyl-N-tert.-butyldimethylsilyltrifluoroacetamide or N-tert.-butyldimethylsilylimidazole were employed to derivatize a
P Bydal et al.
Steroids, 61(6), 349-353 (1996-06-01)
Five dehydrated compounds obtained from a tert-butyldimethylsilylchloride/imidazole or an aqueous hydrochloric acid treatment of 17 alpha-butyl-3-O-methyl estradiol in refluxing solvent were purified and characterized. Three compounds were obtained from a direct vicinal proton elimination, the two others from a vicinal
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service