T4528
Thioglucosidase from Sinapis alba (white mustard) seed
≥100 units/g solid
Synonym(s):
Glucosinolase, Myrosinase, Thioglucoside glucohydrolase
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
CAS Number:
MDL number:
UNSPSC Code:
12352204
NACRES:
NA.54
Recommended Products
biological source
plant seeds (Sinapis alba)
form
solid
specific activity
≥100 units/g solid
storage temp.
−20°C
Related Categories
General description
Thioglucosidase, also called as myrosinase, is present in the myrosin cells that do not contain glucosinolates. This enzyme is obtained from several plant sources, such as Lepidium sativum, L. Sinapis alba and Brassica napus.
Application
Thioglucosidase from Sinapis alba (white mustard) seeds has been used as a standard to quantify myrosinase activity and in column glucosinolate analysis of plant samples.
Thioglucosidase has been used in a study to assess Brassica species screening for glucosinolate content. Thioglucosidase has also been used in a study to investigate a negative regulatory role for auxin in sulphate deficiency response in Arabidopsis thaliana.
Biochem/physiol Actions
Thioglucosidase research has focused mainly on the cruciferous crops due to their economic importance and cancer preventive benefits.
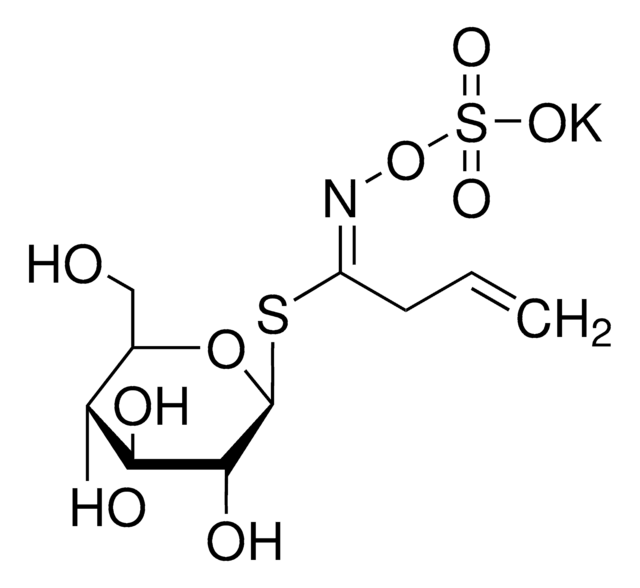
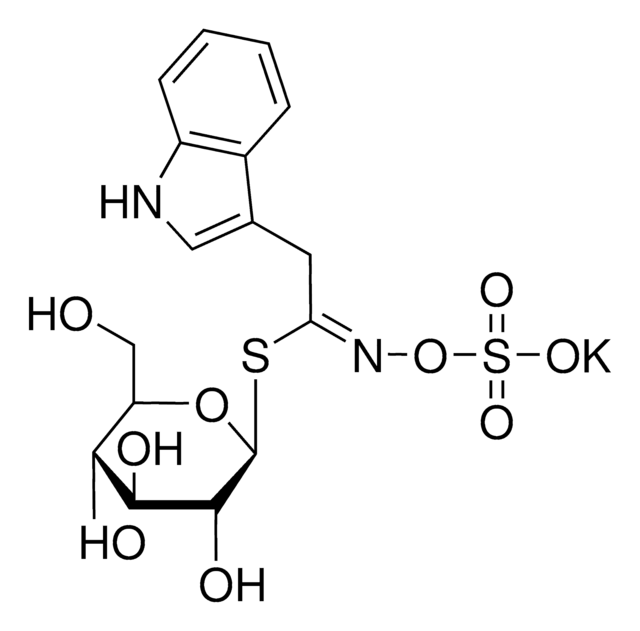
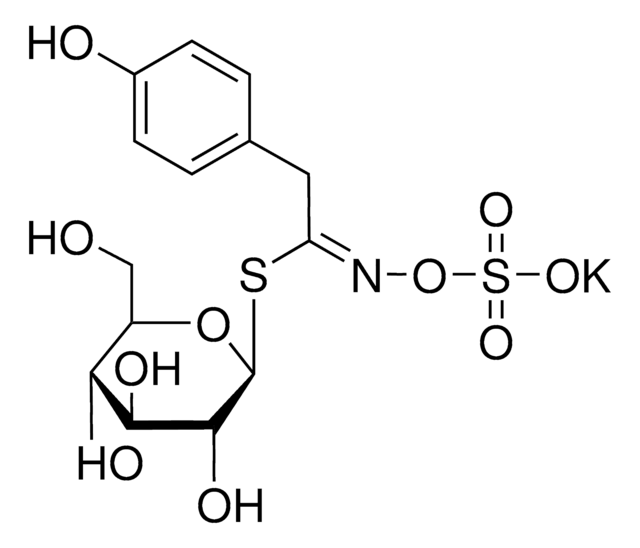
Myrosinases are present in many bacteria, fungi, and edible plants, including those of the Brassicaceae (Cruciferae) family. The enzyme hydrolyzes the S-glucosidic bond of a glucosinolate substrate to form an unstable aglycone that rearranges with the loss of sulfate primarily to the isothiocyanate, though thiocyanates and nitriles are also formed. Many of the isothiocyanate products of aliphatic and aromatic glucosinolates have cancer chemopreventive properties.
Unit Definition
One unit will produce 1.0 μmole glucose per min from sinigrin at pH 6.0 at 25 °C.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Resp. Sens. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Teresa Oliviero et al.
Molecular nutrition & food research, 62(18), e1700837-e1700837 (2018-03-14)
Optimization of bioavailability of dietary bioactive health-beneficial compounds is as important as increasing their concentration in foods. The aim of this study is to explore the change in bioavailability of isothiocyanates (ITCs) in broccoli sprouts incorporated in protein, fiber, and
George F Antonious et al.
Journal of environmental science and health. Part. B, Pesticides, food contaminants, and agricultural wastes, 44(3), 311-316 (2009-03-13)
Glucosinolates (GSLs), a group of compounds found in Brassica plants, are toxic to some soil-borne plant pathogens because of the toxicity of their hydrolysis products, isothiocyanates. Other phytochemicals found in Brassica plants, such as phenols and ascorbic acid, may compliment
Shikha Saha et al.
Molecular nutrition & food research, 56(12), 1906-1916 (2012-10-31)
Sulforaphane (a potent anticarcinogenic isothiocyanate derived from glucoraphanin) is widely considered responsible for the protective effects of broccoli consumption. Broccoli is typically purchased fresh or frozen and cooked before consumption. We compared the bioavailability and metabolism of sulforaphane from portions
Wei Chen et al.
Frontiers in physiology, 13, 1013092-1013092 (2022-11-08)
Glycoside hydrolase family 1 (GH1) members exhibit a broad substrate spectrum and play important roles in insect-plant interactions, such as the defensive β-glucosidase and β-thioglucosidase (so-called myrosinase). However, knowledge about the expression profiling and function of glycoside hydrolase family 1
Niels Agerbirk et al.
Phytochemistry, 153, 79-93 (2018-06-11)
Glucosinolates are found in plants of the order Brassicales and hydrolyzed to different breakdown products, particularly after tissue damage. In Barbarea vulgaris R.Br. (Brassicaceae), the dominant glucosinolate in the investigated "G-type" is glucobarbarin, (S)-2-hydroxy-2-phenylethylglucosinolate. Formation of the nitrile from glucobarbarin
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service