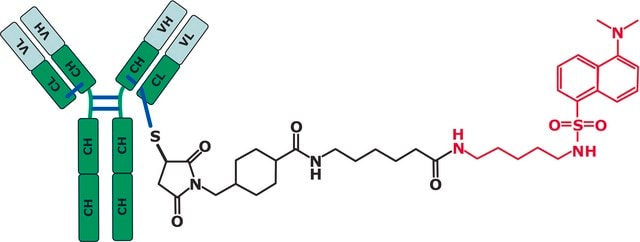
MSQC9
SILu™MAb Infliximab Stable-Isotope Labeled Monoclonal Antibody
recombinant, expressed in CHO cells
Synonym(s):
Mass spectrometry standard, Infliximab, SIL Infliximab, Stable isotope labelled Infliximab
About This Item
Recommended Products
recombinant
expressed in CHO cells
Quality Level
antibody product type
primary antibodies
Assay
≥90% (SDS-PAGE)
packaging
vial of 100 μg
shipped in
wet ice
storage temp.
−20°C
General description
Infliximab has been approved for the treatment of Crohn′s disease, ulcerative colitis, psoriasis, psoriatic arthritis, ankylosing spondylitis, and rheumatoid arthritis. SILu™MAb Infliximab Stable-Isotope Labeled Monoclonal Antibody is for R&D use only. Not for drug, household, or other uses.
Application
Physical form
Preparation Note
Reconstitution
- Briefly centrifuge the vial at ~10,000 × g to collect the product at the bottom of the vial.
- Add 500 μL of ultrapure water containing 0.1% formic acid to the vial.
- Mix the contents by gently inverting the vial a minimum of 5 times.
- Allow the vial to stand at room temperature for at least 15 minutes and repeat mixing by inversion.
Analysis Note
MRM settings provided (xls)
Legal Information
Not finding the right product?
Try our Product Selector Tool.
Storage Class Code
13 - Non Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service