M9400
β-Mannosidase from Helix pomatia
5-30 units/mL, ammonium sulfate suspension, crude extract
Synonym(s):
β-D-Mannoside mannohydrolase
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
CAS Number:
MDL number:
UNSPSC Code:
12352204
NACRES:
NA.32
Recommended Products
form
ammonium sulfate suspension
quality
crude extract
mol wt
94 kDa
concentration
5-30 units/mL
foreign activity
β-N-acetylglucosaminidase and α-mannosidase <1.0%
storage temp.
2-8°C
Looking for similar products? Visit Product Comparison Guide
General description
β-Mannosidase is a lysosomal enzyme that belongs to the glycosyl hydrolase family 2.
Application
β-Mannosidase from Helix pomatia has been used:
- in the digestion of glycans for enzymatic analysis
- in the treatment of labeled monogalactosyl Manno-oligosaccharides
- in non-mammalian glycosidase inhibition assays
Biochem/physiol Actions
β-Mannosidase participates in cleaving the β(1–4)-linked mannose located at the nonreducing end of N-glycosylated proteins. It is involved in the polysaccharide degradation pathway. In humans, mutations in the gene are associated with β-mannosidosis, a lysosomal storage disease. Low levels of β-Mannosidase due to mutations also lead to nystagmus, an eye disorder associated with involuntary eye movements.
Unit Definition
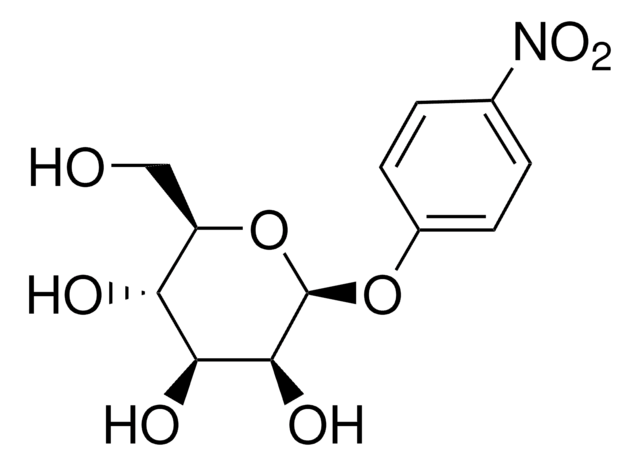
One unit will hydrolyze 1 μmole of p-nitrophenyl-β-D-mannopyranoside to p-nitrophenol and D-mannopyranoside per min at pH 4.0 at 25°C.
Physical form
Suspension in 3.0 M (NH4)2SO4 containing 10 mM sodium acetate, pH approx. 4.0
inhibitor
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
María del Carmen Rodríguez-Gacio et al.
Journal of experimental botany, 63(11), 3976-3988 (2012-05-04)
The softening and degradation of the cell wall (CW), often mannan enriched, is involved in several processes during development of higher plants, such as meristematic growth, fruit ripening, programmed cell death, and endosperm rupture upon germination. Mannans are also the
Yuya Kumagai et al.
Biochimie, 94(12), 2783-2790 (2012-09-27)
Mannanase is an important enzyme involved in the degradation of mannan, production of bioactive oligosaccharides, and biobleaching of kraft pulp. Mannanase must be thermostable for use in industrial applications. In a previous study, we found that the thermal stability of
Guojie Jin et al.
Bioresource technology, 111, 378-382 (2012-03-01)
Lipids produced by oleaginous microorganisms are a potential feedstock for biodiesel production and chemical synthesis. Yet, the costs of microbial lipids remain high, partially because the lipid recovery process is tedious and costly. In the present study, enzyme-assisted extraction of
Jia Zheng et al.
Bioresource technology, 118, 257-264 (2012-06-19)
An industrial medium, Corn Steep Liquor Powder Dextrose (CSD medium) was developed for constitutive expression of recombinant β-mananase by Pichia pastoris. The β-mananase activity (513 U/mL) with CSD medium was 1.64- and 2.5-fold higher than with YPD and BSM in
A P Félix et al.
Journal of animal science, 90(9), 3060-3067 (2012-05-16)
This experiment aimed at evaluating the effects of including the enzyme, β-mannanase, in dog (Canis lupus familiaris) diets based on either poultry (Gallus gallus domesticus) by-product meal (PBM) or soybean [Glycine max (L.) Merr.] Meal (SBM). The second objective was
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service