H6875
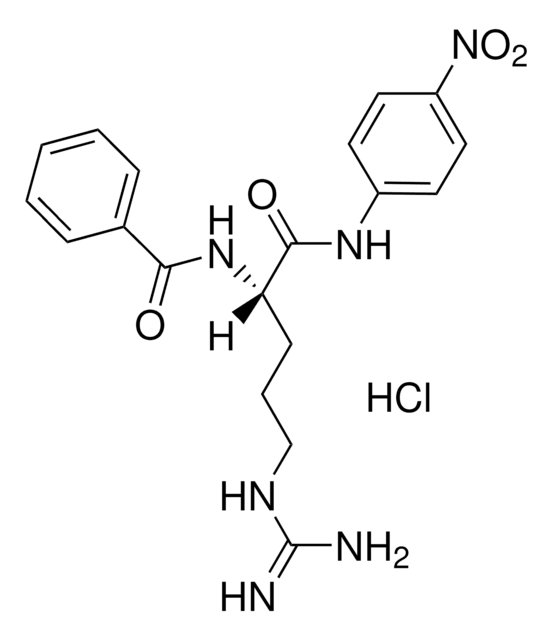
Hippuryl-L-phenylalanine
Synonym(s):
Hippuryl-Phe, N-Benzoyl-Gly-Phe
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C18H18N2O4
CAS Number:
Molecular Weight:
326.35
Beilstein:
2167818
EC Number:
MDL number:
UNSPSC Code:
12352204
PubChem Substance ID:
NACRES:
NA.32
Recommended Products
Assay
≥98% (TLC)
form
powder
solubility
acetic acid: 50 mg/mL, clear, colorless
storage temp.
−20°C
SMILES string
OC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)c2ccccc2
InChI
1S/C18H18N2O4/c21-16(12-19-17(22)14-9-5-2-6-10-14)20-15(18(23)24)11-13-7-3-1-4-8-13/h1-10,15H,11-12H2,(H,19,22)(H,20,21)(H,23,24)/t15-/m0/s1
InChI key
CCLJGZGVIQBNDH-HNNXBMFYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Hippuryl-L-phenylalanine has been used as a substrate for screening carboxypeptidase activity in Trogoderma granarium, Bactrocera oleae Gmelin and Apodiphus amygdali.
Biochem/physiol Actions
Hippuryl-L-phenylalanine is a substrate for carboxypeptidase A enzyme.
Substrates
Substrate for carboxypeptidase A
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Product stabilization of the two forms of carboxypeptidase A from buffalo pancreas
Sud M and Dua RD
Journal of Biosciences, 9(1-2), 83-90 (1985)
J H Cho et al.
Biochemistry, 40(34), 10197-10203 (2001-08-22)
We have investigated the function of Tyr248 using bovine wild-type CPA and its Y248F and Y248A mutants to find that the K(M) values were increased by 4.5-11-fold and the k(cat) values were reduced by 4.5-10.7-fold by the replacement of Tyr248
Digestion and protein metabolism of Trogoderma granarium (Coleoptera: Dermestidae) fed on different barley varieties
Mardani-Talaee M, et al.
Journal of Stored Products Research, 73(1-2), 37-41 (2017)
P W Tardioli et al.
Biotechnology progress, 19(2), 565-574 (2003-04-05)
This paper presents stable carboxypeptidase A (CPA)-glyoxyl derivatives, to be used in the controlled hydrolysis of proteins. They were produced after immobilizing-stabilizing CPA on cross-linked 6% agarose beads, activated with low and high concentrations of aldehyde groups, and different immobilization
Determination of kininase I and kininase II activities in human urine by high-performance liquid chromatography.
G Porcelli et al.
Journal of chromatography, 414(2), 423-428 (1987-03-06)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service