C1999
CMP-Sialic Acid Synthetase from Neisseria meningitidis group B
recombinant, expressed in E. coli BL21, ≥10 units/mg protein
Synonym(s):
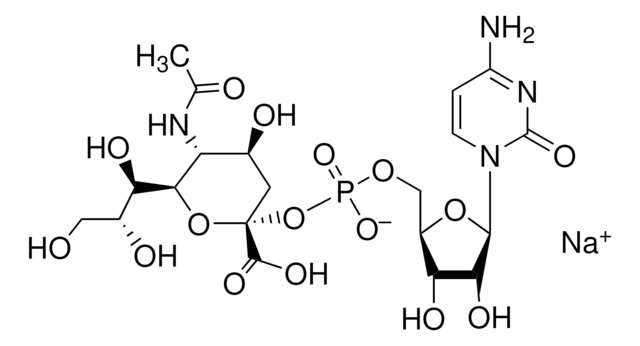
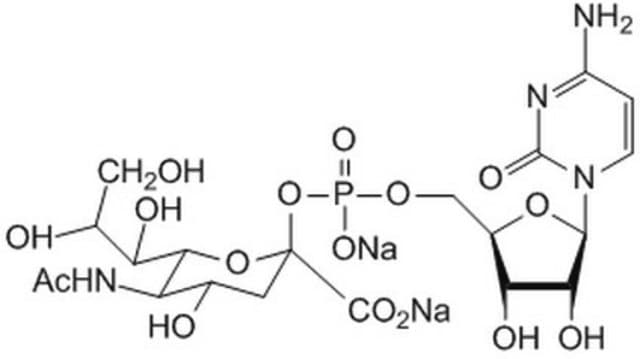
CTP: N-Acylneuraminate cytidylyltransferase
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
MDL number:
UNSPSC Code:
12352204
NACRES:
NA.54
Recommended Products
recombinant
expressed in E. coli BL21
Quality Level
form
lyophilized solid
specific activity
≥10 units/mg protein
mol wt
26.0 kDa
shipped in
dry ice
storage temp.
−20°C
Looking for similar products? Visit Product Comparison Guide
General description
Cytidine monophosphate (CMP)-Sialic Acid Synthetase from Neisseria meningitidis group B is encoded in neuA gene. The protein has a molecular weight of 24.8 kDa.
Application
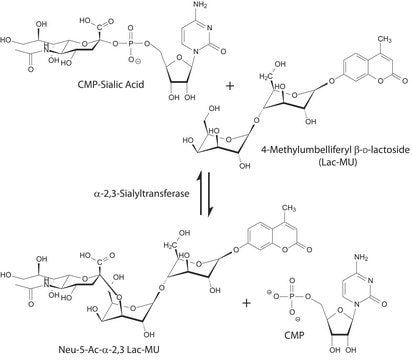
The enzyme has been utilized to synthesize CMP-sialic acid and its derivatives.
Biochem/physiol Actions
Cytidine monophosphate (CMP)-sialic acid synthetase catalyses the conversion of N?acetylneuraminic acid (NeuNAc) to CMP-NeuNAc. CMP-sialic acid synthetase has globular α/β domain and is categorised under αβα three-layered sandwich fold. The dimerization domain aids the interaction between the monomers. It also has mononucleotide binding and NeuAc binding pocket. Mg2+ is essential for the catalytic functionality of CMP-sialic acid synthetase.
Unit Definition
One unit will catalyze the formation of 1 μmol CMP-Neu-5-Ac from Neu-5-Ac and CTP per minute at 37 °C at pH 8.0.
Physical form
Supplied as a lyophilized powder containing Tris-HCl and NaCl.
Analysis Note
Enzymatic activity assays are performed in Tris-HCl buffer (100 mM, pH 8.5) containing Neu-5-Ac (1 mM) and CTP (1 mM) at 37 °C for 30 min and analyzed using capillary electrophoresis with a UV detector (200 nm).
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Rahman M Mizanur et al.
Applied microbiology and biotechnology, 76(4), 827-834 (2007-07-03)
In this study, we report the cloning, recombinant expression, and biochemical characterization of a heat-stable CMP-N-acylneuraminic acid (NeuAc) synthetase from Clostridium thermocellum ATCC 27405. A high throughput electrospray ionization mass spectrometry (ESI-MS)-based assay demonstrates that the enzyme has an absolute
Rahman M Mizanur et al.
Applied microbiology and biotechnology, 80(5), 757-765 (2008-08-22)
Sialic acids are abundant nine-carbon sugars expressed terminally on glycoconjugates of eukaryotic cells and are crucial for a variety of cell biological functions such as cell-cell adhesion, intracellular signaling, and in regulation of glycoproteins stability. In bacteria, N-acetylneuraminic acid (Neu5Ac)
Molecular cloning and analysis of genes for sialic acid synthesis in Neisseria meningitidis group B and purification of the meningococcal CMP-NeuNAc synthetase enzyme.
Ganguli S, et al.
Journal of Bacteriology, 176(15), 4583-4589 (1994)
Jessica H Wong et al.
Organic & biomolecular chemistry, 7(1), 27-29 (2008-12-17)
A modular replacement approach to the synthesis of sulfo-nucleotide analogs prepared from condensation of nucleoside aldehydes with bis phosphonate Horner-Wadsworth-Emmons reagents is disclosed. These analogs were shown to be inhibitors of Neisseria meningitidis CSS (NmCSS), which is a key enzyme
Hai Yu et al.
Bioorganic & medicinal chemistry, 12(24), 6427-6435 (2004-11-24)
Three C terminal His6-tagged recombinant microbial CMP-sialic acid synthetases [EC 2.7.7.43] cloned from Neisseria meningitidis group B, Streptococcus agalactiae serotype V, and Escherichia coli K1, respectively, were evaluated for their ability in the synthesis of CMP-sialic acid derivatives in a
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service