D7600
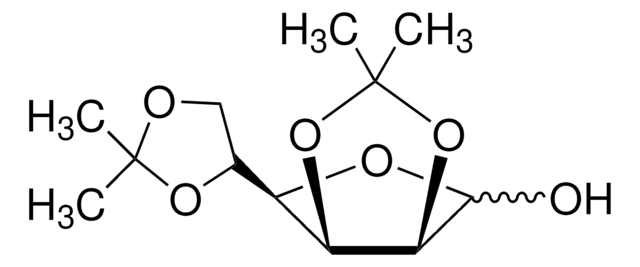
1,2:5,6-Di-O-isopropylidene-α-D-glucofuranose
98%
Synonym(s):
1,2,5,6-diisopropylidene-D-glucose, D-Glucose diacetonide, Diacetone-D-glucose
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C12H20O6
CAS Number:
Molecular Weight:
260.28
Beilstein:
84386
EC Number:
MDL number:
UNSPSC Code:
12352201
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
powder
optical activity
[α]20/D −18°, c = 1% in H2O
mp
109-113 °C (lit.)
SMILES string
[H][C@@]1(O[C@H]2O[C@@H](O[C@@H]2[C@H]1O)C(Cl)(Cl)Cl)[C@H]3COC(C)(C)O3
InChI
1S/C12H20O6/c1-11(2)14-5-6(16-11)8-7(13)9-10(15-8)18-12(3,4)17-9/h6-10,13H,5H2,1-4H3/t6-,7+,8-,9-,10-/m1/s1
InChI key
KEJGAYKWRDILTF-JDDHQFAOSA-N
Application
1,2:5,6-Di-O-isopropylidene-α-D-glucofuranose can be used as a starting material to prepare:
- Biologically active L-acovenose, 6-deoxy-L-idose and, carbanucleoside enantiomers.
- Vinyl ether-based chiral carbohydrate synthon by reacting with acetylene using superbase catalytic systems.
- Fluoro-thiofuranosyl nucleosides of biological importance.
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Direct vinylation of glucose derivatives with acetylene
Trofimov BA, et al.
Tetrahedron, 63(47), 11661-11665 (2007)
An efficient synthesis of 3-fluoro-5-thio-xylofuranosyl nucleosides of thymine, uracil, and 5-fluorouracil as potential antitumor or/and antiviral agents
Tsoukala E, et al.
Bioorganic & Medicinal Chemistry, 15(9), 3241-3247 (2007)
T Z Csáky
Journal of medicine, 16(5-6), 575-586 (1985-01-01)
Based on a previous observation, it was postulated that dimethylsulfoxide (DMSO) acts as a carrier-model in the intestinal absorption of glucose and galactose (Csáky and Ho, 1966). It was further hypothetized that DMSO forms a loosely-bonded hydrophobic complex with the
Z Huang et al.
Methods in molecular biology (Clifton, N.J.), 20, 315-353 (1993-01-01)
Two sets of experimental protocols are given for the synthesis of 3',5'-bis-homodeoxyribonucleosides, building blocks for the synthesis of oligodeoxynucleotide analogs where the -O-PO2-O- groups are replaced by -CH2-S-CH2-, -CH2-SO-CH2-, and -CH2-SO2-CH2- units. Conditions are presented for joining these building blocks
E White et al.
Biomedical mass spectrometry, 9(9), 395-405 (1982-09-01)
Two isotope dilution mass spectrometric methods have been developed for the determination of D-glucose in human serum. Each uses a uniformly labeled (13C)glucose as the internal standard. The first method involves conversion of glucose into 1,2:5,6-di-O-isopropylidene-alpha-D-glucofuranose and an extensive clean-up
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service

![(3AR,5S,6S,6AR)-5-((R)-2,2-DIMETHYL-1,3-DIOXOLAN-4-YL)-2,2-DIMETHYLTETRAHYDROFURO[3,2-D][1,3]DIOXOL-6-OL AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/241/825/1c695a85-5c36-42d3-806a-30876a4dabac/640/1c695a85-5c36-42d3-806a-30876a4dabac.png)