C94009
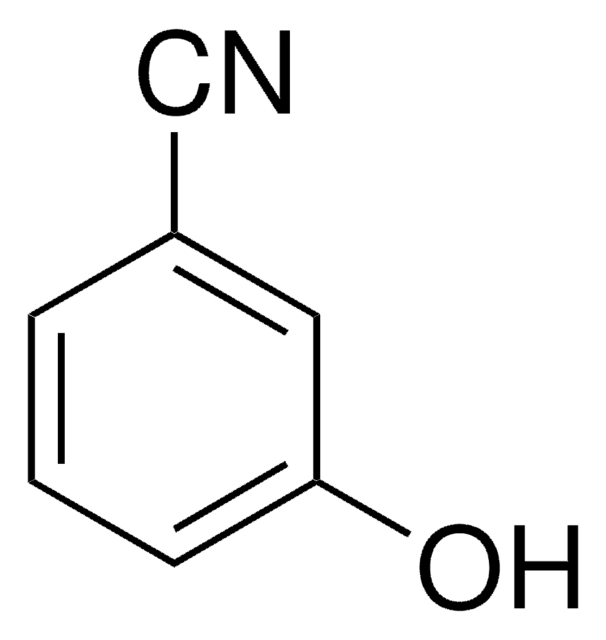
4-Cyanophenol
95%
Synonym(s):
4-Hydroxybenzonitrile
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
NCC6H4OH
CAS Number:
Molecular Weight:
119.12
Beilstein:
386130
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
95%
form
crystals
mp
110-113 °C (lit.)
SMILES string
Oc1ccc(cc1)C#N
InChI
1S/C7H5NO/c8-5-6-1-3-7(9)4-2-6/h1-4,9H
InChI key
CVNOWLNNPYYEOH-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
4-Cyanophenol is a precursor for the synthesis of a vasodilator, Levcromakalim. Bromination of 4-cyanophenol results in bromoxynil, a commercial herbicide. It can also be used as a component of deep eutectic solvent (DES) mixture.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Nitrogen-doped carbons prepared from eutectic mixtures as metal-free oxygen reduction catalysts.
Lopez-Salas N, et al.
Journal of Material Chemistry A, 4(2), 478-488 (2016)
Synthesis and antihypertensive activity of 4-(cyclic amido)-2H-1-benzopyrans.
Ashwood VA, et al.
Journal of Medicinal Chemistry, 29(11), 2194-2201 (1986)
D B Harper
The International journal of biochemistry, 17(6), 677-683 (1985-01-01)
The purification and properties of an enzyme from Nocardia sp. which catalyses the conversion of p-hydroxybenzonitrile to p-hydroxybenzoic acid and ammonia without intermediate formation of the amide is described. The enzyme displayed a broad pH optimum between 7.0 and 9.5
A L Stinchcomb et al.
Pharmaceutical research, 16(8), 1288-1293 (1999-09-01)
Simple, safe and quick in vivo methods for estimating chemical uptake into the stratum corneum (SC) from volatile and non-volatile solvents are invaluable to health risk assessors. This study compares the human in vivo SC uptake of a model compound
Micaela B Reddy et al.
Pharmaceutical research, 19(3), 292-298 (2002-04-06)
Tape stripping the outermost skin layer, the stratum corneum (sc), is a popular method for assessing the rate and extent of dermal absorption in vivo. Results from tape strip (TS) experiments can be affected significantly by chemical diffusion into the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service