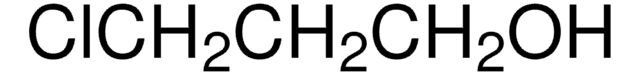All Photos(1)
About This Item
Empirical Formula (Hill Notation):
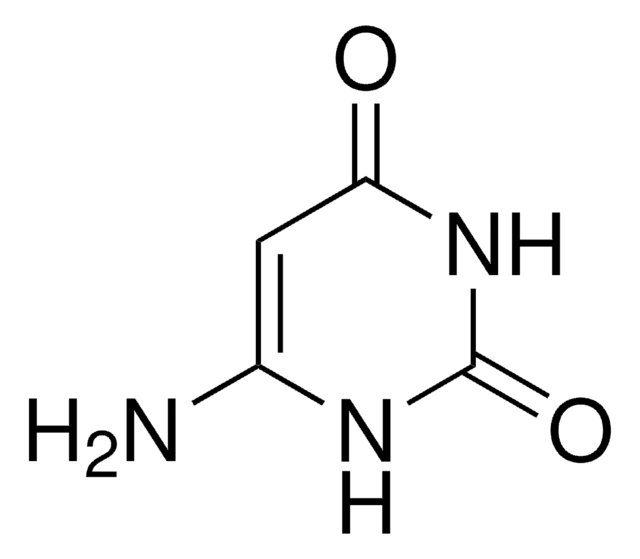
C6H9N3O2
CAS Number:
Molecular Weight:
155.15
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
powder
mp
295 °C (dec.) (lit.)
SMILES string
CN1C(N)=CC(=O)N(C)C1=O
InChI
1S/C6H9N3O2/c1-8-4(7)3-5(10)9(2)6(8)11/h3H,7H2,1-2H3
InChI key
VFGRNTYELNYSKJ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Wafaa S Hamama et al.
Journal of advanced research, 4(2), 115-121 (2013-03-01)
The reaction of 6-amino-1,3-dimethylpyrimidine-2,4(1H,3H)-dione (1) as a binucleophile with primary aromatic or heterocyclic amines and formaldehyde or aromatic (heterocyclic) aldehydes in a molar ratio (1:1:2) gave the pyrimido[4,5-d]pyrimidin-2,4-dione ring systems 2-5. Treatment of 1 with diamines and formalin in molar
Javier Campanini-Salinas et al.
Molecules (Basel, Switzerland), 23(7) (2018-07-22)
A rapid emergence of resistant bacteria is occurring worldwide, endangering the efficacy of antibiotics and reducing the therapeutic arsenal available for treatment of infectious diseases. In the present study, we developed a new class of compounds with antibacterial activity obtained
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service