901138
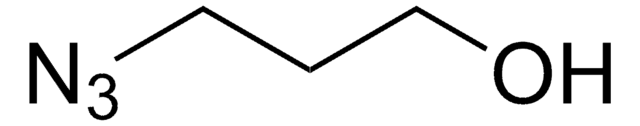
14-Azido-3,6,9,12-tetraoxatetradecan-1-amine
Synonym(s):
2-[2-[2-[2-(2-Azidoethoxy)ethoxy]ethoxy]ethoxy]ethanamine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C10H22N4O4
CAS Number:
Molecular Weight:
262.31
MDL number:
UNSPSC Code:
12352125
NACRES:
NA.22
Recommended Products
form
liquid
Quality Level
reaction suitability
reagent type: linker
refractive index
n/D 1.45
density
1.10 g/mL
functional group
amine
azide
storage temp.
2-8°C
SMILES string
NCCOCCOCCOCCOCCN=[N+]=[N-]
InChI
1S/C10H22N4O4/c11-1-3-15-5-7-17-9-10-18-8-6-16-4-2-13-14-12/h1-11H2
InChI key
ZMBGKXBIVYXREN-UHFFFAOYSA-N
Related Categories
Application
14-Azido-3,6,9,12-tetraoxatetradecan-1-amine is an azide with polyethylene glycol-like characteristics that can be used to prepare fluorescent polymer particles via enzymatic miniemulsion polymerization and copper (I) catalyzed click reaction approach. It is also used as a reactant to synthesize α-aryl-α-diazoamides by aminolysis of N-succinimidyl α-aryl-α-diazoacetates.
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
230.0 °F
Flash Point(C)
110 °C
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
A green approach for the synthesis of fluorescent polymer particles by combined use of enzymatic miniemulsion polymerization with clickable surfmer and click reaction
Kohri Michinari, et al.
Transactions of the Materials Research Society of Japan, 39(1), 57-60 (2014)
Joomyung V Jun et al.
Organic letters, 23(8), 3110-3114 (2021-04-06)
α-Aryl-α-diazoamides were synthesized in two steps under mild conditions. This expeditious route employs Pd-catalyzed C-H arylation of N-succinimidyl 2-diazoacetate to obtain N-succinimidyl 2-aryl-2-diazoacetates, followed by aminolysis. The ensuing diazo compounds can esterify carboxyl groups in aqueous solution, and the ester
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service



![2-[2-(2-Azidoethoxy)ethoxy]ethanol solution ~0.5 M in tert-butyl methyl ether](/deepweb/assets/sigmaaldrich/product/structures/374/007/eea7ca74-41e4-4aac-af71-c93c37ec0a5a/640/eea7ca74-41e4-4aac-af71-c93c37ec0a5a.png)