All Photos(1)
About This Item
Empirical Formula (Hill Notation):
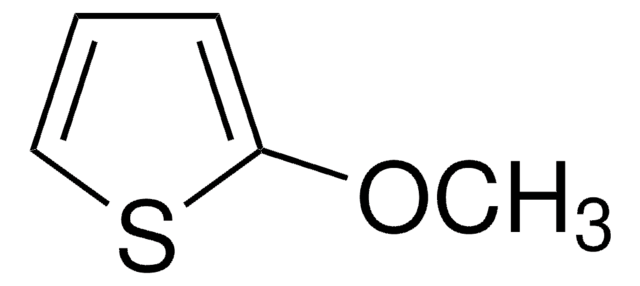
C6H8O2S
CAS Number:
Molecular Weight:
144.19
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
Quality Level
Assay
97%
refractive index
n20/D 1.5409
bp
100-102 °C/10-11 mmHg
density
1.209 g/mL at 25 °C
storage temp.
−20°C
SMILES string
COc1cscc1OC
InChI
1S/C6H8O2S/c1-7-5-3-9-4-6(5)8-2/h3-4H,1-2H3
InChI key
ZUDCKLVMBAXBIF-UHFFFAOYSA-N
General description
3,4-Dimethoxythiophene (DMOT) is a monomer and a precursor which can be synthesized by ring closure reaction of 2,3-dimethoxy-1,3-butadiene and sulfur dichloride in hexane medium. It is an oligothiphene that is majorly used in the development of electroactive materials for organic electronics based applications.
Application
Building block in the synthesis of an N2S2-N4 porphyrin dyad used to study photoinduced energy transfer.
DMOT can be trans-esterified to form 3,4-ethylenendioxythiophene (EDOT). It can further be polymerized to produce PEDOT which can be used as a conductive polymer in π-conjugated systems. It can be polymerized to form poly(dimethoxythiphenes) which can potentially be used in the fabrication energy storage devices on electrochemical doping.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
224.1 °F - closed cup
Flash Point(C)
106.7 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Revisiting the electropolymerization of 3, 4-dimethoxythiophene in organic and micellar media.
Fall M, et al.
Synthetic Metals, 123(3), 365-372 (2001)
Thieno [3, 4-b]-1, 4-oxathiane: An Unsymmetrical Sulfur Analogue of 3, 4-Ethylenedioxythiophene (EDOT) as a Building Block for Linear pi-Conjugated Systems.
Blanchard P, et al.
Organic Letters, 4(4), 607-609 (2002)
Biomimetic Synthesis of Water Soluble Conductive Polypyrrole and Poly (3, 4 ethylenedioxythiophene).
Bruno FF, et al.
MRS Online Proceedings Library, 736(4), 607-609 (2002)
In situ conductance studies of p-and n-doping of poly (3, 4-dialkoxythiophenes).
Skompska M, et al.
Journal of Electroanalytical Chemistry, 577(1), 9-17 (2005)
Sokkalingam Punidha et al.
The Journal of organic chemistry, 73(1), 323-326 (2007-12-12)
Click chemistry has been successfully applied in the synthesis of the first example of a triazole-bridged porphyrin dyad containing N(2)S(2) porphyrin and N(4) or ZnN(4) porphyrin subunits, and fluorescence study indicated a possibility of singlet-singlet energy transfer from the N(4)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service


![2-Chloromethyl-2,3-dihydrothieno[3,4-b]-1,4-dioxine 95%](/deepweb/assets/sigmaaldrich/product/structures/422/187/4cc7b858-9e06-4ce2-8d39-d817b8313964/640/4cc7b858-9e06-4ce2-8d39-d817b8313964.png)



![2,3-Dihydrothieno[3,4-b][1,4]dioxine-5-carboxylic acid AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/145/904/fa7794c7-33ba-4c32-99c7-e9a63b506736/640/fa7794c7-33ba-4c32-99c7-e9a63b506736.png)