All Photos(1)
About This Item
Empirical Formula (Hill Notation):
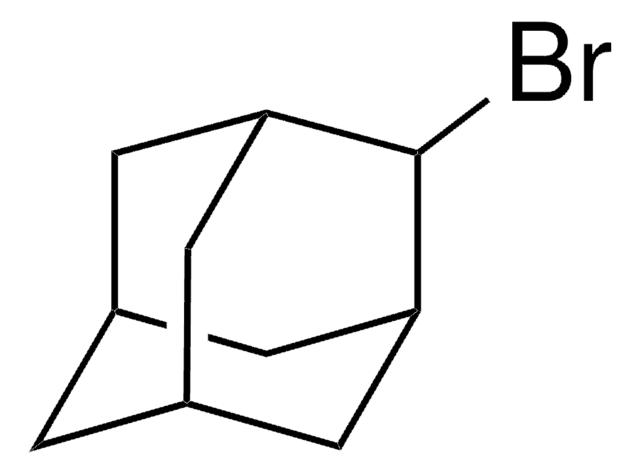
C10H14Br2
CAS Number:
Molecular Weight:
294.03
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
form
powder
mp
108-110 °C (lit.)
SMILES string
Br[C@]12C[C@@H]3C[C@H](C1)C[C@@](Br)(C3)C2
InChI
1S/C10H14Br2/c11-9-2-7-1-8(4-9)5-10(12,3-7)6-9/h7-8H,1-6H2/t7-,8+,9+,10-
InChI key
HLWZKLMEOVIWRK-FIRGSJFUSA-N
Related Categories
General description
1,3-Dibromoadamantane is reported to react with diphenylphosphide ions (Ph2P-) under photostimulation by the SRN1 mechanism. The viscosity-dependent retarding effect of 1,3-dibromoadamantane in highly viscous polymeric chlorotrifluoroethene has been studied by 13C NMR relaxation measurements. Preparation of 1,3-dibromoadamantane via dibromination of adamantine has been reported.
Application
1,3-Dibromoadamantane may be used in the preparation of 1,3-bis(phenyldimethylsilyl)adamantine.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Preparation of 1-silyl-and 1, 3-disilyl-adamantanes.
Pai Y-M, et al.
Journal of Organometallic Chemistry, 270(3), 271-275 (1984)
Lienin et al.
Journal of magnetic resonance (San Diego, Calif. : 1997), 131(2), 184-190 (1998-05-08)
The viscosity-dependent retarding effect of a polymeric solvent on the rotation of small solute molecules is investigated by 13C NMR relaxation measurements. It is found that the relaxation data of 1,3-dibromoadamantane in highly viscous polymeric chlorotrifluoroethene can be explained neither
Selective dibromination of adamantane.
Talaty ER, et al.
J. Chem. Soc. Sect. C, 1902-1903 (1968)
Reactions of 1, 3-dihaloadamantanes with diphenylphosphide ions by the SRN1 mechanism. Competition between intermolecular and intramolecular electron transfer reactions.
Lukach AE, et al.
Journal of Physical Organic Chemistry, 7(11), 610-614 (1994)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service