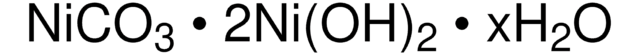377449
Manganese(II) carbonate
≥99.9% trace metals basis
Synonym(s):
Manganese carbonate, Manganese(2+) carbonate, Manganous carbonate
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
MnCO3
CAS Number:
Molecular Weight:
114.95
EC Number:
MDL number:
UNSPSC Code:
12352302
PubChem Substance ID:
NACRES:
NA.23
Assay:
≥99.9% trace metals basis
form:
powder
solubility:
dilute aqueous acid: slightly soluble(lit.)
Recommended Products
Quality Level
Assay
≥99.9% trace metals basis
form
powder
impurities
≤1,000.0 ppm Trace Metal Analysis
mp
>200 °C (lit.)
solubility
dilute aqueous acid: slightly soluble(lit.)
density
3.12 g/mL at 25 °C (lit.)
SMILES string
[Mn++].[O-]C([O-])=O
InChI
1S/CH2O3.Mn/c2-1(3)4;/h(H2,2,3,4);/q;+2/p-2
InChI key
XMWCXZJXESXBBY-UHFFFAOYSA-L
Looking for similar products? Visit Product Comparison Guide
General description
Manganese(II) carbonate is a chemical compound that has a structure similar to calcite, with octahedral co-ordination symmetry. It is a carbonate that is insoluble in water and on treatment with acid it gives water soluble salts. It is a widely used material in plant fertilization as an additive that cures the magnesium deficiency in crops.
Application
Manganese(II) carbonate can be used as:
- A primary electrode material in asymmetric supercapacitors for improving the charge storage capacity and overall performance of the supercapacitors.
- A precursor for synthesizing manganese oxide, which is used as a component in various electrochemical applications, including batteries and supercapacitors.
- A precursor material in the synthesis of oxygen vacancy-rich nitrogen-doped manganese carbonate (MnCO2@N) microspheres. These microspheres are then employed as cathode materials in aqueous zinc-ion batteries (ZIBs) to enhance their electrochemical performance.
- A potential electrocatalyst for the oxygen evolution reaction (OER) in water splitting applications.
Storage Class Code
13 - Non Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Manganese (II) salts as efficient catalysts for chemo selective transesterification of beta-keto esters under non-conventional conditions
Krishnaiah G, et al.
Tetrahedron Letters, 54(7), 703-706 (2013)
Structures of hydrothermally synthesized cobalt (II) carbonate and nickel (II) carbonate
Pertlik F
Acta Crystallographica Section E: Crystallographic Communications, 42(1), 4-5 (1986)
K Maciejewska et al.
Nanomaterials (Basel, Switzerland), 10(3) (2020-03-04)
Herein, a novel synthesis method of colloidal GdPO4:Mn2+,Eu3+ nanoparticles for luminescent nanothermometry is proposed. XRD, TEM, DLS, and zeta potential measurements confirmed the crystallographic purity and reproducible morphology of the obtained nanoparticles. The spectroscopic properties of GdPO4:Mn2+,Eu3+ with different amounts
Jyoti Patel et al.
Nanomaterials (Basel, Switzerland), 10(5) (2020-05-24)
Norfloxacin (NOFX), a broadly used fluoroquinolone antibiotic, has been a subject of great concern in the past few years due to its undesirable effect on human beings and aquatic ecosystems. In this study, novel Mn doped ZnS (Mn:ZnS) quantum dots
Małgorzata Szlachta et al.
Journal of colloid and interface science, 365(1), 213-221 (2011-10-05)
The adsorption behaviour and mechanism of As(III) and Se(IV) oxyanion uptake using a mixed inorganic adsorbent were studied. The novel adsorbent, based on Fe(III)-Mn(III) hydrous oxides and manganese(II) carbonate, was synthesised using a hydrothermal precipitation approach in the presence of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service