All Photos(2)
About This Item
Empirical Formula (Hill Notation):
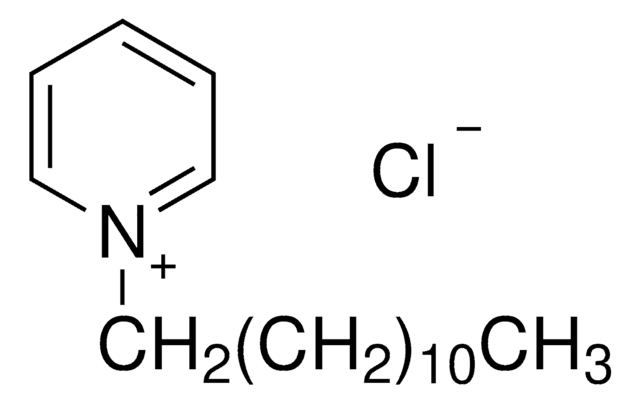
C17H30ClN · xH2O
CAS Number:
Molecular Weight:
283.88 (anhydrous basis)
EC Number:
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
Assay
98%
form
liquid crystal
mp
66-70 °C (lit.)
SMILES string
[Cl-].[H]O[H].CCCCCCCCCCCC[n+]1ccccc1
InChI
1S/C17H30N.ClH.H2O/c1-2-3-4-5-6-7-8-9-10-12-15-18-16-13-11-14-17-18;;/h11,13-14,16-17H,2-10,12,15H2,1H3;1H;1H2/q+1;;/p-1
InChI key
BDGGUWSWAKGEGH-UHFFFAOYSA-M
Related Categories
General description
Dodecylpyridinium chloride is a cationic surfactant.
Application
Micellization characteristics of dodecylpyridinium chloride have been studied. Properties such as molar conductivity, surface tension and surface adsorption parameters has been reported. A non-ionic-cationic mixed system composed of Triton X-100™ and dodecylpyridinium chloride respectively was studied for its clouding behavior.
Legal Information
Triton is a trademark of The Dow Chemical Company or an affiliated company of Dow
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Co- and Counterion Effect on the Micellization Characteristics of Dodecylpyridinium Chloride,
Bhat MA, et al.
Journal of Dispersion Science and Technology, 29(4) null
Synthesis and properties of cationic surfactants with tuned hydrophylicity
Quagliotto P, et al.
Journal of Colloid and Interface Science, 340(2), 269-276 (2009)
Clouding behavior of nonionic?cationic and nonionic?anionic mixed surfactant systems in presence of carboxylic acids and their sodium salts
Jan M, et al.
Coll. Polymer Sci., 285(6), 631- 640 null
D B França et al.
Chemosphere, 242, 125109-125109 (2019-11-02)
Organoclays have been applied as efficient adsorbents for pharmaceutical pollutants from aqueous solution. In this work, dodecylpyridinium chloride (C12pyCl) and hexadecylpyridinium chloride (C16pyCl) cationic surfactants were used for the preparation of organobentonites destined for diclofenac sodium (DFNa) adsorption, an anionic
Daniel Ondo et al.
Journal of colloid and interface science, 505, 445-453 (2017-06-20)
The interaction of α-cyclodextrin (α-CD) with ten ionic surfactants (S) in water was systematically examined using isothermal titration calorimetry. The S comprised cationic and anionic head groups while the hydrocarbon alkyl chain length varied from eight to fourteen carbon atoms.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service