251011
5-Bromo-2,4-dihydroxybenzoic acid monohydrate
98%
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
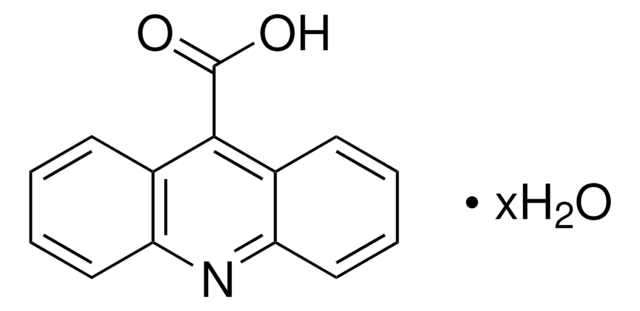
BrC6H2(OH)2CO2H · H2O
CAS Number:
Molecular Weight:
251.03
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
powder
mp
209-210 °C (lit.)
solubility
methanol: soluble 25 mg/mL, clear, colorless to faintly yellow
functional group
bromo
SMILES string
O.OC(=O)c1cc(Br)c(O)cc1O
InChI
1S/C7H5BrO4.H2O/c8-4-1-3(7(11)12)5(9)2-6(4)10;/h1-2,9-10H,(H,11,12);1H2
InChI key
MRIKDYLDHHINRD-UHFFFAOYSA-N
Application
5-Bromo-2,4-dihydroxybenzoic acid has been used as electrolyte for isotachophoresis separation of selenoamino acids (selenoethionine, selenocystine and selenomethionine) on microchips.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Lin Chen et al.
Lab on a chip, 6(4), 474-487 (2006-03-31)
The application of miniaturized total analysis systems (microTAS) has seen rapid development over the past few years. Isotachophoresis (ITP) has been transferred into microchip format for both electrophoretic separation and pretreatment purposes, due to its advantageous features including separation parameters
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service