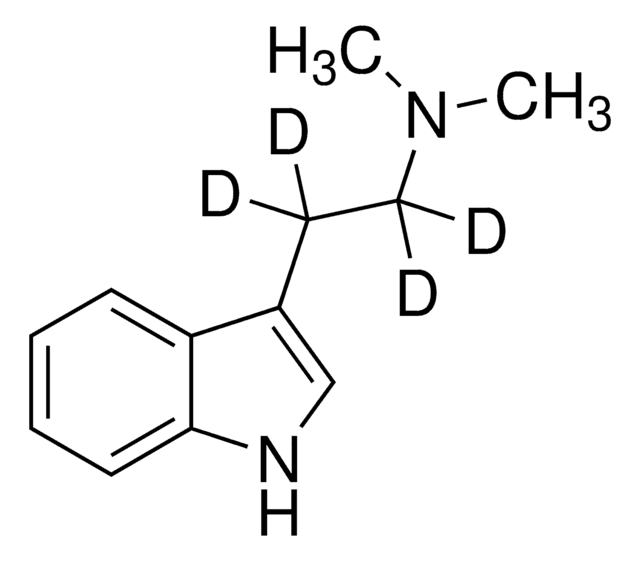
23100
Chloral hydrate
crystallized, ≥98.0% (T)
Synonym(s):
Trichloroacetaldehyde hydrate
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
Cl3CCH(OH)2
CAS Number:
Molecular Weight:
165.40
Beilstein:
1698497
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥98.0% (T)
form
solid
quality
crystallized
mp
57 °C (lit.)
functional group
chloro
hydroxyl
SMILES string
OC(O)C(Cl)(Cl)Cl
InChI
1S/C2H3Cl3O2/c3-2(4,5)1(6)7/h1,6-7H
InChI key
RNFNDJAIBTYOQL-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Chloral hydrate can be used as:
- A reactant in the asymmetric synthesis of β-trichloromethyl-β-hydroxyketones by reacting with chiral imines.
- A water carrier for the efficient deprotectionof acetals, dithioacetals, and tetrahydropyranyl ethers in organic solvents.
- A reactant to synthesize 2-(hydroxyimino)-N-(2-iodophenyl)acetamide using 2-iodoaniline in the presence of hydrochloric acid, sodium sulfate, and hydroxyamine hydrochloride.
Other Notes
Sales restrictions may apply
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Oral - Eye Irrit. 2 - Skin Irrit. 2
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Chloral hydrate as a water carrier for the efficient deprotection of acetals, dithioacetals, and tetrahydropyranyl ethers in organic solvents
Chandrasekhar S, et al.
Synthetic Communications, 44, 1904-1913 (2014)
Jianguo Wang et al.
Journal of agricultural and food chemistry, 59(18), 9892-9900 (2011-08-16)
Acetohydroxyacid synthase (AHAS) catalyzes the first common step in the biosynthesis of the branched-chain amino acids. As a result of its metabolic importance in plants, it is a target for many commercial herbicides. Virtual screening analysis inspired the evaluation of
Kazumasa Funabiki et al.
Organic letters, 5(12), 2059-2061 (2003-06-07)
[reaction: see text] Chloral or its hydrate undergoes the carbon-carbon bond-formation reaction with various optically active imines in the absence of any additive, followed by hydrolysis, to produce the corresponding beta-trichloromethyl-beta-hydroxy ketones in good yields with high enantioselectivities. In addition
Litong Fan et al.
Journal of orthopaedic research : official publication of the Orthopaedic Research Society, 37(6), 1387-1397 (2019-01-16)
Transforming growth factor beta (TGF-β) is commonly utilized in chondrogenic differentiation protocols, but this often results in incomplete maturation of the derived chondrocytes. Gene expression analysis, quantitation of sulfated glycosaminoglycan and collagen, and histological staining were performed to assess the
Stephanie K West et al.
The British journal of ophthalmology, 97(11), 1437-1442 (2013-09-21)
To report the largest study on the safety and effectiveness of sedation in paediatric ophthalmology in a nurse-led outpatient sedation unit. Retrospective cohort study reviewing all children who underwent sedation from January 2006 to December 2010. Patients were sedated with
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service