All Photos(1)
About This Item
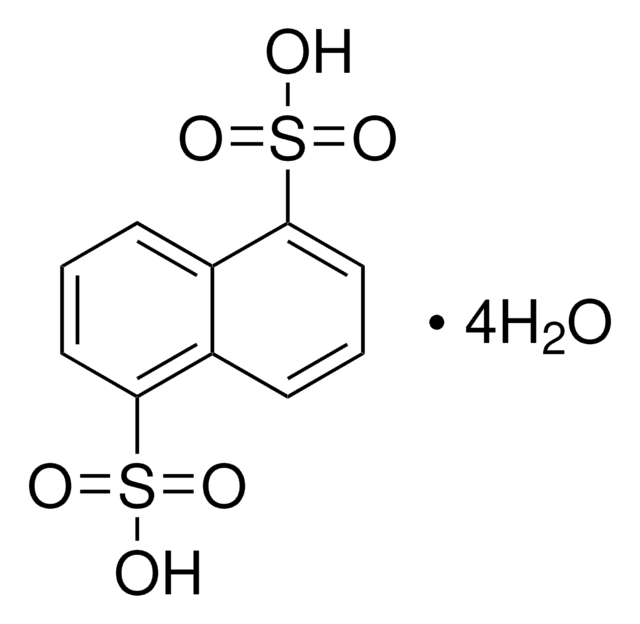
Linear Formula:
C10H7SO3H
CAS Number:
Molecular Weight:
208.23
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
form
solid
concentration
>50%
mp
77-79 °C (lit.)
solubility
alcohol: freely soluble
diethyl ether: slightly soluble
water: freely soluble
SMILES string
OS(=O)(=O)c1cccc2ccccc12
InChI
1S/C10H8O3S/c11-14(12,13)10-7-3-5-8-4-1-2-6-9(8)10/h1-7H,(H,11,12,13)
InChI key
PSZYNBSKGUBXEH-UHFFFAOYSA-N
Gene Information
human ... EGFR(1956) , LCK(3932)
General description
Mechanism of metabolism of 1-naphthalenesulfonic acid by green algae Scenedesmus obliquus has been investigated.
Application
1-Naphthalenesulfonic acid was used as template molecule to prepare new non-covalent molecularly imprinted polymer for solid-phase extraction of naphthalene sulfonates.
Other Notes
remainder naphthalenesulfonic acid, sulfuric acid and water
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Met. Corr. 1 - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
H Kneifel et al.
Archives of microbiology, 167(1), 32-37 (1997-01-01)
Under sulfate limitation, axenic batch cultures of the green alga Scenedesmus obliquus metabolized 1-naphthalenesulfonic acid and partially used the sulfonate as a source of sulfur. The main metabolite, 1-hydroxy-2-naphthalenesulfonic acid, which was not metabolized further in the algal culture, was
Prajna Mishra et al.
The journal of physical chemistry. B, 123(6), 1256-1264 (2019-01-15)
It has been extremely challenging to detect protein structures with a dynamic core, such as dry molten globules, that remain in equilibrium with the tightly packed native (N) state and that are important for a myriad of entropy-driven protein functions.
Yanqing Wang et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 206, 538-546 (2018-09-05)
The investigation about polysaccharides-protein system is attributed to numerous very important applications for pharmaceutical, food, chemical and other industries. In the present work, multi-spectral methods and molecular docking were used to analyze the molecular interactions of polysaccharides from Ganoderma Lucidum
Ester Caro et al.
Journal of chromatography. A, 1047(2), 175-180 (2004-10-06)
A new polymeric sorbent synthesised by exploiting molecular imprinting technology has been used to selectively extract naphthalene sulfonates (NSs) directly from aqueous samples. In the non-covalent molecular imprinting approach used to prepare this polymer, 1-naphthalene sulfonic acid (1-NS) and 4-vinylpyridine
Christina Gkolfinopoulou et al.
Biochimica et biophysica acta. Molecular and cell biology of lipids, 1865(3), 158593-158593 (2019-12-22)
Several hereditary point mutations in human apolipoprotein A-I (apoA-I) have been associated with low HDL-cholesterol levels and/or increased coronary artery disease (CAD) risk. However, one apoA-I mutation, the V19L, recently identified in Icelanders, has been associated with increased HDL-cholesterol levels
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service