185361
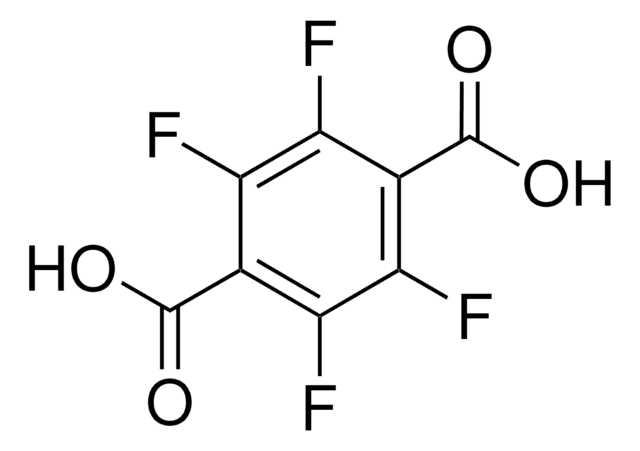
Terephthalic acid
98%
Synonym(s):
Benzene-1,4-dicarboxylic acid
About This Item
Recommended Products
vapor pressure
<0.01 mmHg ( 20 °C)
Assay
98%
form
powder
autoignition temp.
925 °F
greener alternative product characteristics
Design for Energy Efficiency
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
mp
>300 °C (lit.)
solubility
water: ~0.017 g/L at 25 °C
density
1.58 g/cm3 at 25 °C
greener alternative category
SMILES string
OC(=O)c1ccc(cc1)C(O)=O
InChI
1S/C8H6O4/c9-7(10)5-1-2-6(4-3-5)8(11)12/h1-4H,(H,9,10)(H,11,12)
InChI key
KKEYFWRCBNTPAC-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
We are committed to bringing you Greener Alternative Products, which adhere to one or more of The 12 Principles of Greener Chemistry. This product has been enhanced for energy efficiency. Find details here.
Application
- As a monomer in the synthesis of poly(butylene terephthalate) (PBT), a type of polyester, that is used in various fields including Automotive components, textile Industry, packaging materials, electrical and electronic components.
- As an organic ligand in the synthesis of the cobalt(II) metal–organic framework (MOFs), which finds applications in electrochemical energy storage, catalysis, optoelectronics, and water treatment.
- Terephthalic acid (TPA) can be synthesized from bio-based materials for a variety of applications, which include the production of polyester fiber, non-fiber field, PET bottles, synthetic perfumes and medicines.
- Terephthalic acid is used as a linker molecule in the preparation of metal-organic frameworks (MOFs).
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service