144436
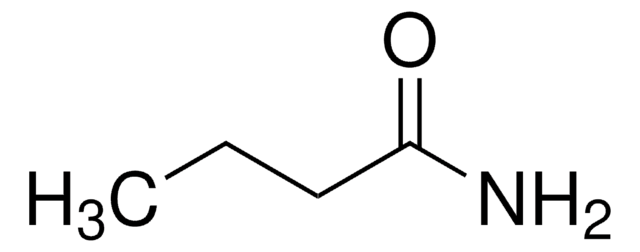
Isobutyramide
99%
Synonym(s):
2-Methylpropionamide
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
(CH3)2CHCONH2
CAS Number:
Molecular Weight:
87.12
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
form
solid
bp
216-220 °C (lit.)
mp
127-131 °C (lit.)
density
1.013 g/mL at 25 °C (lit.)
SMILES string
CC(C)C(N)=O
InChI
1S/C4H9NO/c1-3(2)4(5)6/h3H,1-2H3,(H2,5,6)
InChI key
WFKAJVHLWXSISD-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Isobutyramide was used for chemical grafting of human serum albumin during the synthesis of sequentialy assembled protein capsules.
Biochem/physiol Actions
Isobutyramide activates transcription of human gamma-globin gene and murine embryonic epsilon(y)-globin gene. It is useful in the treatment of β-thalassemia and sickle cell disease.
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
M Domenica Cappellini et al.
Blood cells, molecules & diseases, 26(1), 105-111 (2000-04-25)
A pilot phase II open study on 12 patients with thalassemia intermedia (7 men, 5 women; age 31 +/- 2.0 years SE) treated with oral isobutyramide, a derivative of butyric acid (150 mg/kg body wt/day), was performed in order to
Damien Mertz et al.
Advanced materials (Deerfield Beach, Fla.), 23(47), 5668-5673 (2011-11-22)
Bromoisobutyramide (BrIBAM)-modified silica templates facilitate the formation of bio-functional thin films made of a range of biopolymers (e.g., polypeptides, nucleic acids or polysaccharides). Upon template removal, non-covalent free-standing biopolymeric assemblies (e.g., hollow capsules or replicated spheres and fibers) are formed
M J Haas et al.
Journal of molecular endocrinology, 25(1), 129-139 (2000-07-29)
To determine if ketoacidosis contributes to reduced apolipoprotein A1 (apoA1) expression in insulin-deficient diabetic rats, we examined the regulation of apoA1 gene expression in response to changes in ambient pH or ketone body concentrations. Hepatic apoAI mRNA levels were reduced
G B Strambini et al.
Biochemistry, 29(1), 203-208 (1990-01-09)
The phosphorescence properties of liver alcohol dehydrogenase from horse were characterized at limiting concentrations of coenzyme and coenzyme analogues. The emission decay kinetics of Trp-314 in strong, slowly exchanging, ternary complexes with NADH/isobutyramide, NAD/pyrazole, and NADH/dimethyl sulfoxide displays a markedly
S Reich et al.
Blood, 96(10), 3357-3363 (2000-11-09)
The butyrate derivative isobutyramide (IBT) increases fetal hemoglobin (HbF) in patients with beta-hemoglobinopathies, but little is known about its usefulness for prolonged therapeutic use. We treated 8 patients with transfusion-dependent beta-thalassemia with 350 mg/kg of body weight per day of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service