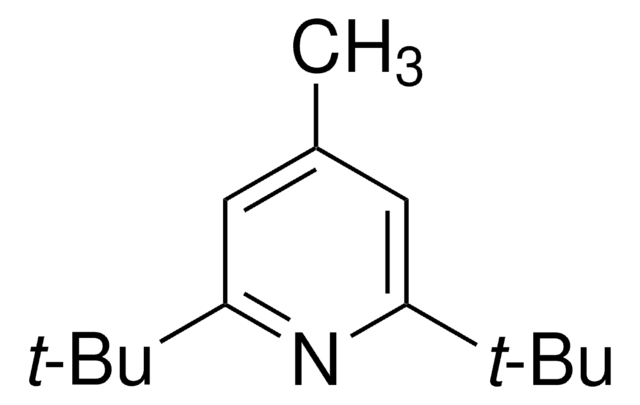
133396
1,2-Dibromotetrachloroethane
97%
Synonym(s):
1,2-Dibromo-1,1,2,2-tetrachloroethane, DBTCE
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
BrCCl2CCl2Br
CAS Number:
Molecular Weight:
325.64
Beilstein:
1699471
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
solid
mp
215-220 °C (dec.) (lit.)
density
2.713 g/mL at 25 °C (lit.)
functional group
bromo
chloro
SMILES string
ClC(Cl)(Br)C(Cl)(Cl)Br
InChI
1S/C2Br2Cl4/c3-1(5,6)2(4,7)8
InChI key
WJUKOGPNGRUXMG-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
1,2-Dibromotetrachloroethane acts as halogenating reagent for the one-pot conversion of sulfones to alkenes by Ramberg-Bäcklund rearrangement. It is a useful brominating reagent for diaryl TeII species.
Application
1,2-Dibromotetrachloroethane was used in quantitative determination of perhalogenated compounds by HPLC.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Stefan C Söderman et al.
The Journal of organic chemistry, 77(23), 10978-10984 (2012-11-14)
Dibromotetrachloroethane (C(2)Br(2)Cl(4)) is demonstrated as a halogenating reagent for the one-pot conversion of sulfones to alkenes by way of the Ramberg-Bäcklund rearrangement. Dibromotetrachloroethane successfully replaces known ozone depleting agents CCl(4), CBr(2)F(2) and C(2)Br(2)F(4). A formal synthesis of E-resveratrol is demonstrated
Quantitative determination of several simple perhalogenated compounds by high-performance liquid chromatography.
Collins CH and Morgano MA.
Journal of Chromatography A, 846(1), 395-399 (1999)
Catalytic oxidation of thiocarbonyl compounds involving the use of 1, 2-Dibrometatrachloroethane as a brominating reagent for diaryl teII species.
Ley SV, et al.
Tetrahedron Letters, 21(8), 1785-1788 (1980)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service






![Chloro[1,3-bis(2,6-diisopropylphenyl)imidazol-2-ylidene]copper(I)](/deepweb/assets/sigmaaldrich/product/structures/199/763/44637b2e-b87c-42a3-abc3-3985b6cd7d5d/640/44637b2e-b87c-42a3-abc3-3985b6cd7d5d.png)