115819
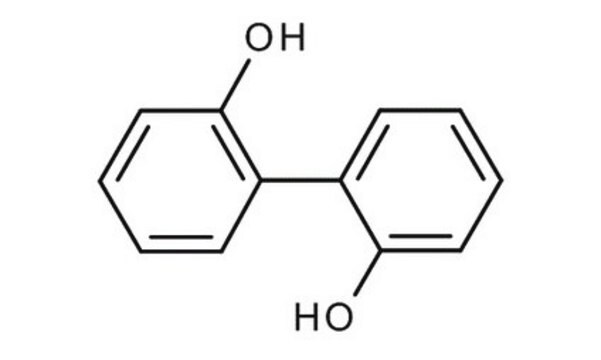
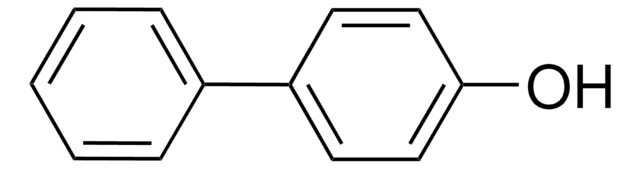
2,2′-Biphenol
99%
Synonym(s):
2,2′-Biphenyldiol, 2,2′-Dihydroxybiphenyl, 2,2′-Diphenol
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
HOC6H4C6H4OH
CAS Number:
Molecular Weight:
186.21
Beilstein:
1638363
EC Number:
MDL number:
UNSPSC Code:
12162002
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
Quality Level
Assay
99%
form
solid
bp
315 °C (lit.)
mp
108-110 °C (lit.)
SMILES string
Oc1ccccc1-c2ccccc2O
InChI
1S/C12H10O2/c13-11-7-3-1-5-9(11)10-6-2-4-8-12(10)14/h1-8,13-14H
InChI key
IMHDGJOMLMDPJN-UHFFFAOYSA-N
Related Categories
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
309.2 °F - closed cup - (External MSDS)
Flash Point(C)
154 °C - closed cup - (External MSDS)
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
T Kuhnigk et al.
Journal of basic microbiology, 37(3), 205-211 (1997-01-01)
The capability of the intestinal flora from the gut of xylophagous termites of degrading lignin model compounds was investigated. Different dimeric lignin model compounds-degrading bacteria were obtained from the hindgut flora of Mastotermes darwiniensis Froggatt, Reticulitermes santonensis Feytaud, Nasutitermes nigriceps
H P Kohler et al.
Applied and environmental microbiology, 54(11), 2683-2688 (1988-11-01)
Pseudomonas sp. strain HBP1 was found to grow on 2-hydroxy- and 2,2'-dihydroxy-biphenyl as the sole carbon and energy sources. The first step in the degradation of these compounds was catalyzed by an NADH-dependent monooxygenase. The enzyme inserted a hydroxyl group
Alaa S Amin et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 96, 541-547 (2012-07-07)
A solid phase extraction technique is proposed for preconcentration and speciation of chromium in natural waters using spectrophotometric analysis. The procedure is based on sorption of chromium(III) as 4-(2-benzothiazolylazo)2,2'-biphenyldiol complex on dextran-type anion-exchange gel (Sephadex DEAE A-25). After reduction of
M Sondossi et al.
Applied and environmental microbiology, 70(1), 174-181 (2004-01-09)
The purpose of this investigation was to examine the capacity of the biphenyl catabolic enzymes of Comamonas testosteroni B-356 to metabolize dihydroxybiphenyls symmetrically substituted on both rings. Data show that 3,3'-dihydroxybiphenyl is by far the preferred substrate for strain B-356.
Bernd Schmidt et al.
The Journal of organic chemistry, 78(17), 8680-8688 (2013-08-01)
User-friendly protocols for the protecting group-free synthesis of 2,2'-biphenols via Suzuki-Miyaura coupling of o-halophenols and o-boronophenol are presented. The reactions proceed in water in the presence of simple additives such as K2CO3, KOH, KF, or TBAF and with commercially available
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service